More Information
Submitted: 25 September 2020 | Approved: 20 October 2020 | Published: 21 October 2020
How to cite this article: Wang L, Li Z, Tan C, Wang H, Zhou Z, et al. Lower-body negative pressure/ergometer exercise in bed rest: Effects on female orthostatic tolerance. J Nov Physiother Rehabil. 2020; 4: 040-048.
DOI:10.29328/journal.jnpr.1001036
Copyright License: © 2020 Wang L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Head-down bed rest; Lower-body negative pressure; Orthostatic intolerance; Exercise; Tilt testc
Abbreviations: ALD: Aldosterone; ANGII: Angiotensin II; ANOVA: Analysis Of Variance; AVP: Arginine Vasopressin; BMI: Body Mass Index; BP: Blood Pressure; BR: Bed Rest; CO: Cardiac Output; CON: Control Group; DBP: Diastolic Blood Pressure; ECG: Electrocardiogram; E2: Estradiol; Ergo: Ergometer; Hb: Hemoglobin; HCT: Hematocrit; HDBR: Head-Down Bed Rest; HR: Heart Rate; LBNP: Lower Body Negative Pressure; MSV: Mean Of SV; NASA: National Aeronautics And Space Administration; NE: Norepinephrine; OI: Orthostatic Intolerance; POTS: Postural Orthostatic Tachycardia Syndrome; PV: Plasma Volume; RAAS: Renin-Angiotensin-Aldosterone System; R-2: 2 Days Before Bed Rest; R+1: The Day Immediately After 15 Days Of Bed Rest; R+5: 5 Days After Bed Rest; SBP: Systolic Blood Pressure; SD: Standard Deviation; SE: Standard Error; SV: Stroke Volume; TPR: Total Peripheral Vascular Resistance; VO2peak: Peak Oxygen Uptake
Lower-body negative pressure/ergometer exercise in bed rest: Effects on female orthostatic tolerance
Linjie Wang1*, Zhili Li1, Cheng Tan1, Huijuan Wang1, Xiangjie Zhou2, Siyang He1, Peng Zou1 and Yinghui Li1
1State Key Laboratory of Space Medicine Fundamentals and Application, China Astronaut Research and Training Center, Beijing 100094, China
2Space City Clinic, Beijing 100094, China
*Address for Correspondence: Linjie Wang, State Key Laboratory of Space Medicine Fundamentals and Application, China Astronaut Research and Training Center, Beijing 100094, China, Tel: 86-10-68746545; Fax: 86-10-68746545; Email: [email protected]
Introduction: Alternatively using gradient lower-body negative pressure (LBNP) and ergometer exercise (LBNP + ergo) under a flight schedule framework was explored to detect its orthostatic capacity maintenance effects in female subjects after 15 days of -6° head-down bed rest (HDBR).
Methods: Twenty-two female university students were divided into a control group (n = 8), an LBNP group (n = 7), and an LBNP + ergo group (n = 7). Ergometer exercise consisted of an interval exercise protocol with 2 min intervals alternating between 41% and 70% VO2max. Gradient LBNP was decompressed in 10 mm Hg intervals to -40 mmHg every 5 min. intermittent ergometer exercise and LBNP were alternatively performed. Tilt test was performed 2 days before HDBR (R-2), on the day of HDBR termination (R+1), and 5 days after HDBR (R+5).
Results: Fifty percent of the participants (11/22) did not pass the tilt test on R+1. The orthostatic tolerance time decreased from 20 to 16.1 ± 2.1 min in the control group, to 10.0 ± 2.7 min in the LBNP group (p = 0.01) and to 16.3 ± 2.0 min in the LBNP + ergo group. The HRs and BPs were at similar level among three groups during tilt test on different test days. Compared with the control group, the LBNP + ergo group had higher SV and CO percentage changes at R+1(p < 0.023) and R+5 (p < 0.00001) during the tilt test.
Conclusion: LBNP combined with ergometer exercises fails to prevent orthostatic intolerance but it induced some positive hemodynamic changes during tilt test after 15 days HDBR.
Cardiovascular deconditioning during space flight induces postflight orthostatic intolerance (OI). Astronauts are unable to maintain normal blood pressure in upright posture and result in lightheadedness, diaphoresis, nausea, syncope and etc [31]. Approximately 20% of astronauts who return from short-term space flights and 83% of those returning from long-term flights experience postflight OI [23]. In addition, female astronauts are 22% - 61% more susceptible to postflight OI than male astronauts [15]. A lower vascular resistance response, greater dependence on volume status, and smaller stroke volume (SV) secondary to a smaller and less compliant left ventricle are the potential underlying mechanisms for this condition [6,15]. The mechanisms underlying the difference in susceptibility between the two genders are females’ inherent lower center of gravity, larger mass in the lower extremities [28], greater decrease in blood pressure, smaller increase in plasma norepinephrine, decreased baroreflex sensitivity, smaller stroke volume, sex hormones such as estrogens and progesterone involved in blood pressure control [1,2,31]. However, the exact gender difference mechanisms have not been elucidated thus far and are likely multifactorial.
At the National Aeronautics and Space Administration (NASA), ~22% of the active astronaut corps are women. On average, female astronauts are 10 cm shorter and 13 kg lighter, and they have 11% more body fat, 8% less muscle mass, 10% - 14% less haemoglobin mass, and lower level of aerobic fitness than their male counterparts [15]. In China, 2 out of 10 (20%) active astronaut corps are women. On average, these female astronauts are similar in height to their male counterparts but are ~10 kg lighter. These characteristic differences with gender may influence their exercise capacity, and therefore, the results of countermeasures performed during space flight may differ.
Thus far, at least four microgravity studies involving -6° head-down bed rest (HDBR) have included female subjects. Greenleaf et al. studied 12 female subjects aged 23–34 years who participated in a 17-day HDBR study [10]. Goldenberg, et al. studied eight female subjects aged 28–34 years who participated in a 13-day HDBR study [7]. In 2005, 24 female subjects aged 25–40 years participated in a WISE study involving 60 days of HDBR [26]. In 2007, seven pairs of female twins aged 24.4 ± 2.6 (mean ± SD) years participated in a 30-day HDBR study [33]. These experiments investigated the physiological effects of simulated weightlessness and countermeasures for it. A major difference between our study and the forementioned studies is that we recruited Chinese female subjects aged 19–24 years, as compared to Caucasian female subjects aged 23-40 years in those studies.
Lower-body negative pressure (LBNP) mimics changes in the circulatory dynamics of 1 G standing and is used to counteract postflight OI [9,21]. Exercising increases the plasma volume, muscle mass and, when at an appropriate intensity, is beneficial for improving OI [26,32]. Ergometer exercise is usually used as an endurance training method to counter the negative effects of simulated microgravity and to maintain cardiopulmonary and lower-leg muscle functions [14,24]. Exercise performed under LBNP has been widely studied as an integrated countermeasure to simulate the effects of Earth-like loads on the musculoskeletal system, gravitational vascular transmural pressures and fluid distribution [25]. Previous studies have found that a daily 40-min run with LBNP fails to protect orthostatic tolerance after 15 days of HDBR, and a 40 min/day of supine LBNP treadmill exercise followed immediately by 5 mins of rest improves, but does not fully alleviate, OI associated with 30 days of bed rest (BR) [25,30]. Jeong, et al. found that supine cycling plus volume loading prevents OI after 18 days of HDBR [18]. In manned space flights, space is limited for large exercise equipment. Furthermore, in space, treadmill use under LBNP is more difficult than the combination of independent LBNP and treadmill use. For our short-duration space flight, the ergometer is the assembled aerobic training device instead of treadmill. Thus far, it remains unclear whether a combination of LBNP and ergometer exercise (LBNP + ergo) has similar effects on OI as treadmill under LBNP. Therefore, this study aimed to determine the physiological effects of simulated microgravity and the countereffects of LBNP + ergo on OI in young Chinese female subjects. In addition, we attempted to illustrate the difference between the simultaneous countereffects and combined countereffects on OI between the two countermeasure modalities. The determined effects of LBNP + ergo will provide guidance for medical monitoring techniques for Chinese female astronauts in short-duration space flights and have potential use in the nonpharmacological treatment of postural orthostatic tachycardia syndrome (POTS) in clinical settings.
Subjects
Advertisements to recruit candidates were published on the China Agriculture University website and at the Beijing Institute of Technology. The candidates were screened and recruited by clinic personnel at China’s Space City. All study protocols were reviewed and approved by the ethics committee of the Astronaut Center of China. All subjects received verbal and written explanations of all BR and test protocols before they provided informed consent. Subjects were free to withdraw from the study, and they were financially rewarded for their participation.
Subjects were excluded because they had one of the following conditions: infectious disease, abnormal electrocardiogram (ECG), menalgia, body mass index (BMI) <18 kg/m2 or >26 kg/m2, history of spinal trauma, inherent vestibular system disorders, dermatitis, or a family history of mental stress. Of the 80 female university students who were screened, 22 candidates aged 21 ± 0.4 (mean ± SE) years were finally selected. No subjects were on birth-control pills.
A routine blood chemistry test and urinalysis were performed on all selected subjects. Before BR, the subjects’ menstrual periods were recorded, and peak oxygen uptake (VO2peak) was estimated by a submaximal oxygen consumption test using a -6° head-down ergometer to balance the fitness level between groups.
Table 1 presents the characteristics of all three groups pre- and post-HDBR. The groups did not differ significantly in terms of body weight, height, or submaximal oxygen uptake prior to HDBR. The VO2peak was at a relatively low level due to the -6 head-down test mode. The submaximal oxygen uptake decreased in the control (CON) and LBNP groups but remained the same in the LBNP + ergo group after 15 days of HDBR.
| Table 1: Characteristics of the subjects. | ||||||
| CON | LBNP | LBNP+ergo | ||||
| Characteristics | Pre-BR | Post-BR | Pre-BR | Post-BR | Pre-BR | Post-BR |
| Age (yrs) | 21.6 ± 1.4 | - | 22.0 ± 1.4 | - | 19.7 ± 0.8 | - |
| Weight (kg) | 52.8 ± 6.5 | 52.3 ± 6.8 | 52.5 ± 6.5 | 50.5 ± 3.3 | 55.8 ± 2.7 | 54.9 ± 2.6 |
| Height (cm) | 160.8 ± 7.3 | 162.2 ± 7.9 | 161.6 ± 3.5 | 160.2 ± 3.4 | 161.1 ± 2.4 | 161.2 ± 2.6 |
| VO2peak (ml×kg-1min-1) | 27.5 ± 4.7 | 25.6 ± 4.0* | 26.3 ± 2.7 | 23.3 ± 2.7** | 26.3 ± 4.2 | 25.1 ± 5.1 |
| *,vs pre-BR, p < 0.05; **, vs pre-BR, p < 0.01 | ||||||
Experimental design
The subjects were matched according to their VO2peak values and then randomly divided into three groups: BR-only group (n = 8, CON: subjects were exposed to -6° HDBR); BR LBNP group (n = 7, LBNP: subjects were given LBNP during -6° HDBR); and BR LBNP combined with ergometer exercise countermeasure group (n = 7, LBNP + ergo: subjects alternatively exercised with ergometer or exposed to LBNP). The pre-BR phase included 7 days for acclimation and baseline data collection, followed by 15 days of BR and 7 days of post-BR testing and reconditioning.
The subjects’ menstrual cycles were recorded but not controlled before and during HDBR (menstrual period distribution: pre-HDBR: 5 subjects, during HDBR: 8 subjects, post-HDBR: 9 subjects). All subjects have normal menstrual cycle and period (3 to 5 days). Estrogen levels indicator estradiol (E2) were monitored, and no significant differences were detected before and after 15-day HDBR (E2: 47.8 ± 7.5 pg/ml vs. 43.3 ± 4.5 pg/ml, p = 0.592). None of the subjects underwent endurance training before the experiment.
Countermeasure protocol
The countermeasures used in this experiment were -6° head-down ergometer exercise and supine LBNP chamber that targeted countereffects in the cardiovascular system and muscle function.
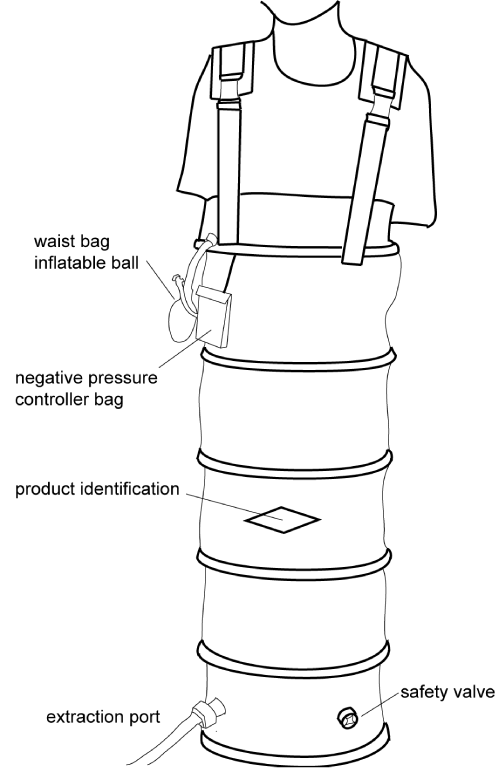
Figure 1 is a schematic diagram of the LBNP chamber. It was a flight-simulation device (maximum negative pressure: 50 mmHg; time to reach the maximum: <10 min) that was sealed at the midabdominal level. LBNP consisted of 5-min intervals of suction at -10, -20, -30 and -30 mmHg in the first 2 trials. In the third trial, the negative pressures were increased to -20, -20, -30 and -40 mmHg of suction. In the last two trials, LBNP included 5-min intervals of -20, -20, -30 and -40 mmHg suction, which were then slowly (1 min) recovered to 0 mmHg, and the subjects were given additional 10- min intervals of -20, -30, -40 mmHg and 5 mins of -30 mmHg suction.
Figure 1: A schematic diagram of the LBNP chamber, LBNP, lower-body negative pressure.
The ergometer exercises were conducted with moderate-intensity interval training and consisted of 3 mins of warm-up at 40% pre-BR VO2peak, followed by 6 × 2 min stages at 60%, 65%, 70%, 75%, 80%, and 75% VO2peak. The 6 stages were separated by 2 mins of active rest stages at 50% VO2peak, and the last stage was a 3-min cool-down at 40% VO2peak. The VO2peak for each subject was determined with a -6° head-down, graded ergometer test before HDBR. The power outputs (W) used during the ergometer exercise were calculated from a linear regression of oxygen consumption and exercise intensity results from the pre HDBR VO2peak test. Exercise protocol did not change during the menstrual cycle.
Given the constraints of rendezvous, docking operations and item sorting before returning, countermeasure usage should be assigned from day 4 to day 13 in a 15-day mission. For the LBNP + ergo group, LBNP was given during HDBR on days 6, 8, 10, 12 and 13, and the ergometer exercise was performed on days 4, 5, 7, 9 and 11. The total LBNP cumulative stimulation intensity was 4650 mmHg×min. The LBNP group underwent LBNP on the same day as the LBNP + ergo group and served as LBNP control. Heart rate (HR) was continuously monitored by a bedside ECG monitor during LBNP and ergometer exercise. Auscultation blood pressure (BP) was measured every 5 mins during LBNP. The Borg subjective fatigue scale (7—very easy, 20—exhaustion) was used in the ergometer exercise, and scores above 18 led to a 25 W power reduction in the exercise. The LBNP termination criteria included rapidly exhibiting symptoms such as excessive sweating, pallor, vertigo, nausea or a sudden drop in BP (systolic blood pressure (SBP) of > 25 mmHg/min or diastolic blood pressure (DBP) decrease of > 15 mmHg/min or SBP < 70 mmHg) or HR (HR decrease of > 15 beats/min) [13].
Procedures
The subjects were positioned at exactly -6° HDBR for a duration of 15 days. A tilt test was used for measurements at the following time points: 2 days before BR (R-2), the day immediately after 15 days of BR (R+1), and 5 days after BR (R+5). The tilt test on R+1 was the first test after BR. Before the tilt test, the subjects rested in the supine position and were not allowed to get up. The test was conducted from 0900 h to 1130 h and from 1400 h to 1730 h. The tilt test schedule for each subject was the same before and after HDBR. During HDBR, subjects performed all activities while lying in bed, including having meals, washing, bathing, and urinating (on paper diapers), except for a 10-min defecation period daily. The subjects’ postures in bed were monitored using a video-surveillance system, and they were permitted to change their body position along the longitudinal axis. Bedside stand frameworks were provided for the subjects to mount their personal laptops, and they were allowed to use these laptops for 2 to3 hours during the resting period. Two or three subjects shared one room, and their beds were separated by a movable curtain. The daily time schedule was as follows: wake up at 0600 h, breakfast from 0700 to 0730 h, lunch from 1200 to 1230 h, dinner from 1800 to 1900 h, and sleep from 2200 to 0600 h. At 2200 h, the room lights were turned off to maintain a consistent day-night cycle. Each night, the staff in charge visited the rooms 2 to 3 times to monitor the subjects’ postural positions.
Tilt test
Subjects were transferred to the test room by a transport stretcher for the tilt test and were maintained in supine position for 10 mins. Subsequently, they were tilted to 75° in ~5 s to begin 20 mins of 75° tilt standing. Thereafter, they were allowed 5 mins for supine recovery. During the test, ECG signal was recorded using the ECG channel of a Holter system (Medilog95, Oxford, Great Britain), and the beat-to-beat BP was monitored continuously using Portapres (noninvasive Finapres Medical System, Netherlands). The SBP, DBP, cardiac output (CO), SV, and total peripheral vascular resistance (TPR) were determined by analyzing the beat-to-beat BP waveforms using BeatScope software (FMS, Finapres Medical Systems BV, Amsterdam, Netherlands). Parameters such as weight, height, age, and sex were input into Portapres before recording the data. Through the Modelflow method used in the BeatScope software, derived parameters SV, CO, TPR were presented as percentage changes in comparison to the 5-min supine resting mean values in consideration of the limitations of the method for computing the absolute values [27]. Hemodynamic variables were averaged in each group over each minute during the tilt standing course. The percentage changes of these parameters at R+1 or R+5 tilt test relative to the respective value at R-2 tilt test were used to perform statistical analysis.
Computation of ∆SV% during the tilt test at R+1 was given below as an example.
SVi % = [(SVi − MSVsupine) / MSVsupine] ×100 (1)
SVi −− SV at i min during tilt standing; MSVsupine −− the mean of SV in the 5- min supine; SVi % −− SV percentage change at i min during tilt test.
∆SVi-R+1 % = SVi-R+1 % − SVi-R+2 % (2)
SVi-R+1 % −− SV percentage changes at i min during tilt standing at R+1; SVi-R+2 % −− SV percentage change at i min during tilt standing at R-2; ∆SVi-R+1 % −− SV percentage change difference at i min during tilt standing at R+1.
∆SVi-R+1 % or ∆SVi-R+5 % was the data used to perform statistical analysis. ∆SV%, ∆CO% and ∆TPR% were computed in the same mode.
Before and after the tilt test, 5 mL of blood sample was collected from each subject, and arginine vasopressin (AVP), norepinephrine (NE), angiotensin II (ANGII), and aldosterone (ALD) levels were measured.
Blood volume estimation
The Dill & Costil equation was used to calculate the changes in plasma volume (PV) [4].
∆PV=(PVpost-PVpre)/PVpre= Hbpre(1-HCTpost)/Hbpost(1-HCTpre) – 1 (3)
Hb—hemoglobin concentration; HCT—hematocrit.
Hb and HCT were measured in venous blood samples taken from the antecubital vein of the left arm before HDBR and 3 days after HDBR.
Statistical analysis
A repeated-measure analysis of variance was used to perform the statistical analysis for each minute of hemodynamic data collected during the tilt test. The R project was used for statistics and analyses (download from https://mirrors.tuna.tsinghua.edu.cn/CRAN/). The within - subjects factor was test time (two levels: R+1, R+5). The between - subjects factor was group (three levels: CON, LBNP and LBNP + ergo). The Geisser – Greenhouse correction was used when the assumed sphericity was not approved, and the Tukey’s test was used to give multiple comparisons. The normality of variables was tested using the Kolmogorov–Smirnov test. Due to the onset of presyncope symptoms, the number of subjects completing the tilt test post-HDBR decreased over time in each group, therefore, data were collected from 5 subjects per group and hemodynamic variables were compared every minute. Hormone blood concentrations of the renin – angiotensin - ALD system (RAAS) before and after the tilt on different testing days also underwent a repeated-measure analysis of variance. The within - subjects factors were test time (three levels: R-2, R+1, R+5) and tilt time (two levels: pretilt, post-tilt). The between-subjects factor was group (three levels: CON, LBNP and LBNP + ergo). Student’s paired t test was used to compare orthostatic tolerance time before and after bed rest at R+1 in three groups, and one-way analysis of variance (ANOVA) was used to compare ∆PV. Survival proportions of the three groups in the tilt test immediately after HDBR were subjected to a log-rank test. Values are presented as the mean ± standard error. Statistical significance in all comparisons was set at p < 0.05.
Implementation of countermeasure protocols
Subjects in the LBNP and LBNP + ergo groups completed 100% of the prescribed ergometer exercises and LBNP regimens. Most of the subjects in the LBNP group complained about noise during LBNP action and that too much time was taken for the seal state preparation.
Orthostatic tolerance time
All subjects passed the tilt test before BR, and only 1 subject in the CON group did not pass the tilt test 5 days after HDBR. In addition, this participant was in the first 2 days of her menstrual cycle. Immediately after HDBR (recovery day 1), 50% (11/22) of the subjects did not pass the tilt test. In 6 cases, the test ended because of sudden drops in SBP and was accompanied by decreases in HR in 3 subjects in the CON group, 1 subject in the LBNP group and 2 subjects in the LBNP + ergo group. In the other 5 cases, the test ended due to sudden, rapid falls of HR, associated with drops in BP in 4 subjects in the LBNP group and 1 subject in the LBNP + ergo group. A significant decrease in the orthostatic tolerance time (min, mean ± SE) immediately after HDBR was found in the LBNP group (CON: 20 versus 16.1 ± 2.1, NS; LBNP: 20 versus 10.0 ± 2.7, p = 0.010; LBNP+ ergo: 20 versus 16.3 ± 2.0, NS). There was no significant difference in orthostatic tolerance time between groups.
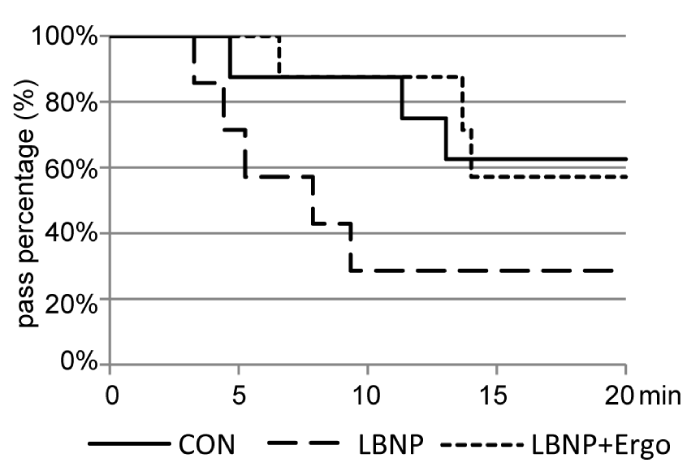
The survival curve of the tilt test immediately after BR (Figure 2) demonstrated that 5 out of 8 subjects in the CON group (62.5%), 2 out of 7 subjects in the LBNP group (28.6%) and 4 out of 7 subjects in the LBNP + ergo group (57.1%) finished 20 mins of tilt standing. Although the survival of the LBNP + ergo group was greater than that of the CON group in the first 14 mins of tilt standing, no significant differences in survival were detected among the three groups.
Figure 2: Survival curve of the tilt test at R+1 in the CON, LBNP, and LBNP + ergo groups. CON: Control; LBNP: Lower-Body Negative Pressure; Ergo, Ergometer The solid line represents the CON group; the dotted line represents the LBNP group; the dashed line represents the LBNP + ergo group.
Hemodynamic variables during tilt tests
Mean values of HR and BP during the tilt test at R-2, R+1 and R+5 were calculated and plotted in the CON, LBNP and LBNP + ergo groups. Mean values of SV, CO or TPR changes at R+1 or R+5 in relative to the values at R-2 during the tilt test were calculated and plotted. Curves were drawn if there were at least 5 subjects per group.
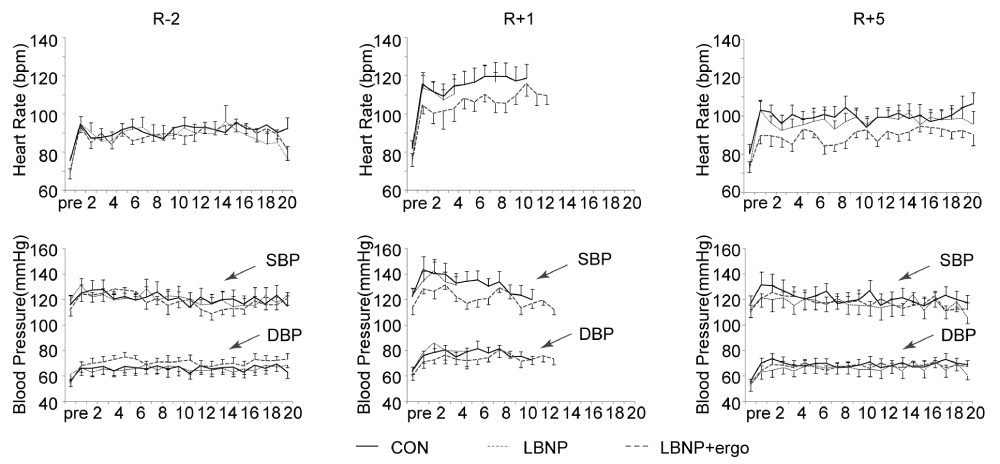
HR the HR and BP during the tilt tests in the three groups are illustrated in figure 3. Pre HDBR at R-2, the HR (bpm, mean±SE) showed no significant differences among the three groups at rest in the supine position (82 ± 7 in CON, 78 ± 3 in LBNP and 75 ± 3 in LBNP + ergo). Post-HDBR at R+1 (82 ± 5 in CON, 78 ± 4 in LBNP and 75 ± 3 in LBNP + ergo) and at R+5 (82 ± 6 in CON, 77 ± 3 in LBNP and 72 ± 3 in LBNP + ergo), the HR also did not significantly differ in the supine position between groups. Post-HDBR at R+1, the HR was relatively similar at pre-syncope for the three groups (136 ± 4 in CON, 129 ± 4 in LBNP and 128±1 in LBNP + ergo).
Figure 3: HR, SBP and DBP during the tilt test at R-2, R+1, and R+5 in the CON, LBNP, and LBNP+ergo groups. CON, control; LBNP, lower-body negative pressure; ergo, ergometer; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; R-2, 2 days before BR; R+1, on the day of HDBR termination; R+5, 5 days after HDBR. The solid line represents the CON group; the dashed line represents the LBNP group; the dotted line represents the LBNP + ergo group.
The HR during the tilt test from 1 min to 13 min at R+1 increased significantly compared to that at R-2 (main effects of HDBR, p < 0.03). No significant group effects or group × HDBR interactions were detected.
BP (Figure 3) SBP and DBP were not significantly affected by HDBR in the supine position or during the course of the tilt and were not different between groups. Before HDBR, the SBP and DBP (mmHg, mean ± SE) in the three groups at supine rest were similar (mean values of SBP/DBP were 116 ± 7/56 ± 4 for CON, 119 ± 5/61 ± 5 for LBNP and 112 ± 5/54 ± 3 for LBNP + ergo). During the tilt course, the BP was also not significantly different among the three groups on different test days (mean values of SBP/DBP at R-2, R+1 and R+5 were 121 ± 3/66 ± 1, 128 ± 3/78 ± 1, 122 ± 5/69 ± 4 for the CON group, 121 ± 1/66 ± 1, 131 ± 3/77 ± 2, 117 2/66 ± 2 for the LBNP group and 119 ± 2/71 ± 1, 121 ± 2/74 ± 1, 118 ± 2/67 ± 1 for the LBNP + ergo group).
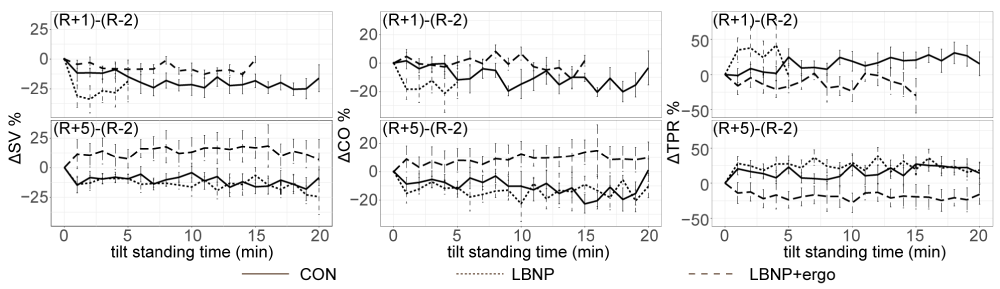
∆SV% (Figure 4) The ∆CO%, ∆SV% and ∆TPR% at R+1 or R+5 across the tilt standing course are presented in figure 4. A significant test time effect (F [1, 616] = 56.8835; p < 0.00001) and group effect (F [2, 616] = 71.861; p < 0.00001) were detected in ∆SV%. An interaction between group and test time was also presented (F [2, 616] = 7.864; p < 0.0004). The ∆SV% in CON group was lower than that in LBNP + ergo group at R+1 (p < 0.004) and the ∆SV% in CON and LBNP group were significantly lower than that in LBNP + ergo group at R+5 (p < 0.00001).
Figure 4: SV%, CO% and TPR% changes during the tilt test at R+1 and R+5 in the CON, LBNP, and LBNP+ergo groups. CON, control; LBNP, lower-body negative pressure; ergo, ergometer. SV, stroke volume; CO, cardiac output; TPR, total peripheral vascular resistance. R+1, on the day of HDBR termination; R+5, 5 days after HDBR. ΔSV%, SV% in R+1 or R+5 minus SV % in R-2. ΔCO%, CO% in R+1 or R+5 minus CO% in R-2. ΔTPR%, TPR% in R+1 or R+5 minus TPR% in R-2. The solid line represents the CON group; the dotted line represents the LBNP group; the dashed line represents the LBNP + ergo group. The SV% in CON group was lower than that in LBNP + ergo group at R+1 (p < 0.004) and the SV% in CON group and LBNP was significantly lower than that in LBNP + ergo group at R+5 (p < 0.00001). The CO% in CON group was lower than that in LBNP + ergo group at R+1 (p < 0.023) and the CO% in CON group and LBNP was significantly lower than that in LBNP + ergo group at R+5 (p < 0.00001).
∆CO% (Figure 4) a significant group effect (F [2, 616] = 55.025; p < 0.00001) was detected in ∆CO%. An interaction between group and test time was also presented (F [2, 616] = 4.780; p < 0.009). The ∆CO% in CON group was lower than that in LBNP + ergo group at R+1 (p < 0.023) and the ∆CO% in CON and LBNP group were significantly lower than that in LBNP + ergo group at R+5 (p < 0.00001).
∆TPR% (Figure 4) a significant group effect (F [2, 616] = 86.960; p < 0.00001) was detected in ∆TPR%. The ∆TPR% in CON and LBNP group was significantly higher than that in LBNP + ergo group (p < 0.00001). The ∆TPR% in CON group was lower than that in LBNP group (p < 0.013).
Neuroendocrine fluid regulation
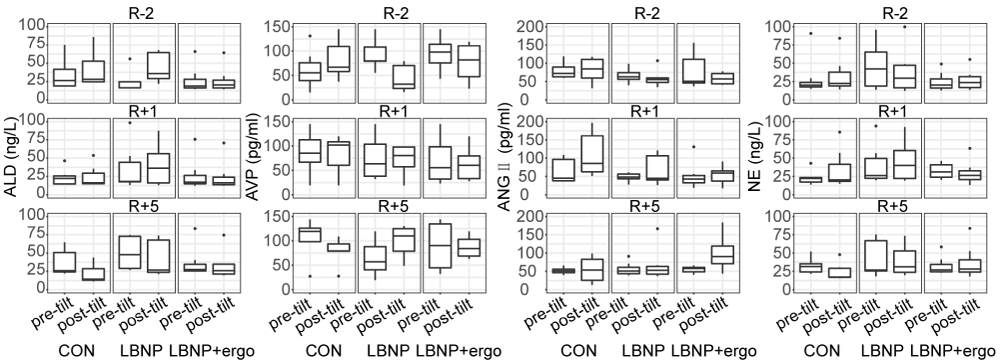
Renin-angiotensin-aldosterone system (RAAS) The RAAS system hormone blood concentrations before and after the tilt test on different testing days are depicted in figure 5. A significant group effect was detected in ALD (F [2, 114] = 7.297; p < 0.001) and NE (F [2, 114] = 8.576; p < 0.0004). The level of ALD and NE were lower in LBNP + ergo group than that in LBNP group (p < 0.002). A significant test time effect (F [2, 114] = 4.683; p < 0.02) and tilt time effect (F [1, 114] = 6.447; p < 0.02) were detected in ANGII. The level of ANGII increased significantly post-tilt than pretilt (p < 0.02) and decreased significantly at R+5 in comparison to R-2 (p < 0.009).
Figure 5: The level of ALD, AVP, ANGII and NE of the CON, LBNP, and LBNP+ergo groups before and after tilt test performed at R-2, R+1 and R+5. ALD: Aldosterone; AVP: Arginine Vasopressin; ANGII: Angiotensin II; NE: Norepinephrine. CON: Control; LBNP: Lower-Body Negative Pressure; Ergo, Ergometer. R-2, 2 days before HDBR; R+1, on the day of HDBR termination; R+5, 5 days after HDBR. The level of ALD and NE were lower in LBNP + ergo group than LBNP group (p < 0.002). The level of ANGII increased significantly post-tilt than pretilt (p < 0.02) and decreased significantly at R+5 in comparison to R-2 (p < 0.009).
Blood volume
The changes in plasma volume (%, mean ± SE) 3 days after HDBR showed no significant differences among the groups (CON: 7.9 ± 3.8%; LBNP group: 8.7 ± 5.7%; LBNP + ergo group: 9.8 ± 5.4%).
In this study, LBNP combined with ergometer exercise failed to protect women from OI after 15 days of HDBR, but induced some positive hemodynamic responses when challenged by upright posture. A lower intensity of LBNP deteriorated the orthostatic tolerance after 15 days HDBR.
There are at least two reasons for the failure of LBNP combined with ergometer exercise. The first is the completely random experimental design used in this study. In a similar 15-day BR held by NASA, self-controlled trial design was adopted [25]. In another female HDBR study, identical twins were used as subjects, with one sibling assigned as the control subject and the other as the countermeasure subject [30]. A completely random experimental design cannot overcome the statistical limitations in this experiment, that is, the small sample size and large individual differences. The second reason is the relatively mild orthostatic challenge after BR. In our experiment, the tilt test had a 75° tilt angle and a maximal 20-min duration. This protocol is more easily tolerated than that used in some experiments: 60° tilt angle, 60-min tolerance duration [11] or 80° tilt angle, 30-min tolerance duration [5].
We compared the physiological variables between LBNP combined with ergometer exercise and treadmill exercise in LBNP on OI countereffects after 15 days of HDBR. Schneider et al. conducted a self-controlled 15-day HDBR to explore the OI countermeasure effects of 40 min/day of supine moderate-intensity treadmill exercise inside the LBNP chamber with 50–60 mmHg negative pressure [25]. The OI was evaluated by increasing the LBNP by 10 mmHg every 3 mins. After BR LBNP tolerance decreased in both control and exercise conditions, but the sub-tolerance index HR decreased, and SBP was better maintained in the exercise group than in the control group. In the present study, we used the classic tilt test to evaluate the OI, and we found that at R+1, the SV and CO percentage changes relative to the values at R-2 was higher than that in control group during the tilt test.
We also compared the exercise protocol during the 15-day HDBR. In Schneider’s study, the subjects performed 6 days of exercise, with 40 min/day of supine treadmill exercise against LBNP per week [25]. The intensity of their supine treadmill interval exercise protocol was from 41%~65% VO2max, similar to that of the supine cycling interval exercise protocol used in this study, 41% ~ 70% VO2peak. However, the total exercise duration differed, being 40 mins in Schneider, et al.’s study and 30 mins in our study. As the subjects in Schneider, et al.’s study had relatively higher aerobic capacity (56.44–58.76 ml.kg-1min-1) than the female subjects in this study (27.5–26.3 ml.kg-1min-1 in head-down posture), it can be deduced that the protocol was made to fit the subjects’ condition. In Schneider, et al.’s study, to produce a 1 g foot force, the researchers used -50 to -60 mmHg LBNP [25,30]. In the present study, as our LBNP controller controlled the negative pressure in 10 mmHg intervals and could not be set to 5 mmHg intervals, a modified Russian LBNP action protocol was used. Instead of a maximum negative pressure of 45 mmHg, the maximum negative pressure we used was 40 mmHg [19]. It was deduced that an increase in exercise frequency for LBNP combined with ergometer exercise in this experiment would achieve similar results as those with a moderate-intensity treadmill exercise performed under LBNP.
In this study, the OI countereffects of LBNP were not detected. These factors are as follows: 1) Lower cumulative stimulation intensity (4650 mmHg × min). Until now, at least three HDBR studies have reported the OI countereffects of LBNP. The first experiment used 4 hoursof LBNP at 30 mmHg and saline loading at the end of 1 week of BR (50,400 mmHg × min) and found the protective role of LBNP in OI after HDBR [17]. Sun et al. found that 1 hour of LBNP at 30 mmHg daily in the first and last week (25,200 mmHg × min) of a 21-day HDBR could counteract OI induced by the HDBR [29]. Güell used 3 sessions of 20-min LBNP at 28 mmHg in the first three weeks, and in the last week, 4 sessions of 20-min LBNP at 28 mmHg on the first 4 days and 6 sessions on the last 3 days (54,320 mmHg × min) during 28 days HDBR [12]. All of these experiments used a larger amount of LBNP stimulation than our experiment. 2) Incompletely established LBNP counter effects. The specifications of our LBNP cylinder were as follows: the time of decompression to 50 mmHg is less than 10 mins, and the seal location was at the midabdominal level. The main action of LBNP was the decrease in cardiac filling and the increase in peripheral resistance [20]. The repeated slower onset of this LBNP during the graded LBNP protocol made the neural response of TPR compensate for the decrease in SV (maintained CO and BP) and the neural response is relatively unstable. According to the results from Hisdal and Goswanmi [16], the slower and not rapid speed of LBNP, the midabdominal not iliac crest sealing location in our LBNP chamber [8], and the skewed LBNP response for the subject’s feet resting on the base of the device [9], may lead to a relatively slow establishment of LBNP countereffects.
Limitations
Although we did not find significant differences in E2 levels before and after bed rest, the not controlled menstrual cycle had relatively large impact on the results. The hormonal levels during the menstrual cycle may alter the sympatho-excitation during an orthostatic challenge [3]. Furthermore, even though the hormonal fluctuations during the menstrual cycle did not influence submaximal and maximal exercise performance [22], the subjects’ physiological condition could have had a negative impact on their exercise or LBNP condition.
In summary, the protocol used in this experiment of LBNP combined with ergometer exercise failed to counter the OI after 15 days of HDBR. The cumulative stimulus amount of LBNP is vital to assure its OI countereffect. To reach a similar OI counter effects with treadmill exercise performed under LBNP, the exercise frequency or intensity for LBNP combined with ergometer exercise should increase. The mechanism underlying the effects of this exercise combination still needs further study.
This work was supported by the National Natural Science Foundation of China [grant number 81571845] and Key Project of Logistics Research [grant number BWS14C024]. We would like to thank Wenjuan Chen, Lifen Zhang and Guohua Tang for the technical, medical and psychological support in this experiment. We thank the native English-speaking scientists of American Journal Experts for editing our manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81571845] and Key Project of Key Project of State Key laboratory of Space Medicine Fundamentals and Application [grant number SYFD180011801].
- Barantke M, Krauss T, Ortak J, Lieb W, Reppel M, et al. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J Cardiovasc Electrophysiol. 2008; 19: 1296-1303. PubMed: https://pubmed.ncbi.nlm.nih.gov/18662181/
- Cheng YC, Vyas A, Hymen E, Perlmuter LC. Gender differences in orthostatic hypotension. Am J Med Sci. 2011; 342: 221-225. PubMed: https://pubmed.ncbi.nlm.nih.gov/21289499/
- Carter JR, Lowrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab. 2009; 297: E85-E91. PubMed: https://pubmed.ncbi.nlm.nih.gov/19401460/
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974: 37: 247-248. PubMed: https://pubmed.ncbi.nlm.nih.gov/4850854/
- Dychman DJ, Sauder CL, Ray CA. Effects of short-term and prolonged bed rest on the vestibulosympathetic reflex. Am. J. Physiol. Heart Circ. Physiol. 2012; 302: H368-H374. PubMed: https://pubmed.ncbi.nlm.nih.gov/22021328/
- Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, et al. Hemodynamics of orthostatic intolerance: implications for gender difference. Am. J. Physiol. Heart Circ. Physiol. 2004; 286: H449-H457. PubMed: https://pubmed.ncbi.nlm.nih.gov/14527942/
- Goldberger AL, Mietus JE, Rigney DR, Wood ML, Fortney SM. Effects of head-down bed rest on complex heart rate variability: response to LBNP testing. J Appl Physiol. 1994; 77: 2863-2869. PubMed: https://pubmed.ncbi.nlm.nih.gov/7896633/
- Goswami N, Grasser E, Roessler A, Schneditz D, Hinghofer-Szalkay H. The cardiovascular response to lower body negative pressure in humans depends on seal location. Physiol Res. 2009; 58: 311-318. PubMed: https://pubmed.ncbi.nlm.nih.gov/18637716/
- Goswami N, Blaber AP, Hinghofer-Szalkay H, Convertino VA. Lower body negative pressure: physiological effects, applications and implementation. Physiol Rev. 2019; 99: 807-851. PubMed: https://pubmed.ncbi.nlm.nih.gov/30540225/
- Greenleaf JE, Stinnett HO, Davis GL, Kollias J, Bernauer EM. Fluid and electrolyte shifts in women during +Gz acceleration after 15 days’ bed rest. J Appl Physiol. 1977; 42: 67-73. PubMed: https://pubmed.ncbi.nlm.nih.gov/833079/
- Greenleaf JE, Wade CE, Leftheriotis G. Orthostatic responses following 30-day bed rest deconditioning with isotonic and isokinetic exercise training. Aviat Space Environ Med. 1989; 60: 537-542. PubMed: https://pubmed.ncbi.nlm.nih.gov/2751583/
- Güell A, Cornac A, Faurat MM, Gauquelin G, Pavy-Le-Trano A, et al. Lower body negative pressure as a countermeasure against orthostatic intolerance for long term space flight. Acta Astronautica. 1992; 27: 103-107. PubMed: https://pubmed.ncbi.nlm.nih.gov/11537573/
- Guinet P, Schneider SM, Macias BR, Watenpaugh DE, Hughson RL. et al. WISE-2005: effect of aerobic and resistive exercises on orthostatic tolerance during 60 days bed rest in women. Eur J Appl Physiol. 2009; 106: 217-227. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2881341/
- Guyton AC, Hall JE. Text book of medical physiology (11th ed.). Elsevier Inc: Philadelphia, Pennsylvania. 2006.
- Harm DL, Jennings RT, Meck JV, Powell MR, Putcha L, et al. Invited review: gender issues related to space flight: a NASA perspective. J Appl Physiol. 2001; 91: 2374-2383. PubMed: https://pubmed.ncbi.nlm.nih.gov/11641383/
- Hisdal J, Toska K, Walloe L. Beat-to-beat cardiovascular responses to rapid, low-level LBNP in humans. Am J Physiol Regulatory Integrative Comp Physiol. 2001; 281: R213-R221. PubMed: https://pubmed.ncbi.nlm.nih.gov/11404296/
- Hyatt KH, West DA. Reversal of bedrest-induced orthostatic intolerance by lower body negative pressure and saline. Aviat Space Environ Med. 1977; 48: 120-124. PubMed: https://pubmed.ncbi.nlm.nih.gov/871280/
- Jeong SM, Shibata S, Levine BD, Zhang R. Exercise plus volume loading prevents orthostatic intolerance but not reduction in cerebral blood flow velocity after bed rest. Am J Physiol Heart Circ Physiol. 2012; 302: H489-H497. PubMed: https://pubmed.ncbi.nlm.nih.gov/22081705/
- Kozlovskaya IB, Pestov ID, Egorov AD. The system of preventive measures in long space flights. Human Physiology. 2010; 36: 773-779.
- Ligtenberg G, Blankestijn PJ, Koomans HA. Hemodynamic response during lower body negative pressure: role of volume status. J Am Soc Nephrob. 1988; 9: 105-113. PubMed: https://pubmed.ncbi.nlm.nih.gov/9440094/
- Louisy F, Gaudin C, Oppert JM, Güell A, Guezennec CY. Haemodynamics of leg veins during a 30-day -6° head-down bed rest with and without lower body negative pressure. Eur J Appl Physiol. 1990; 61: 349-355.
- Mattu MT, Iannetta D, MacInnis MJ, Doyle-Baker PK, Murias JM. Menstrual and oral contraceptive cycle phases do not affect submaximal and maximal exercise responses. Scand J Med Sci Sports. 2020; 30: 472-484. PubMed: https://pubmed.ncbi.nlm.nih.gov/31663173
- Meck JV, Reyes CJ, Perez SA, Goldberger AL, Ziegler MG. Marked exacerbation of orthostatic intolerance after long- vs. short-duration spaceflight in veteran astronauts. Psychosom Med. 2001; 63: 865-873. PubMed: https://pubmed.ncbi.nlm.nih.gov/11719623/
- O’Hara R, Khan M, Pohlman R, Schlub J. Leg resistance training: effects upon VO2peak and skeletal muscle myoplasticity. Am J Exerc Physiologists. 2004; 7: 27-43.
- Schendier SM, Watenpaugh DE, Lee SMC, Ertl AC, Williams WJ, et al. Lower-body negative-pressure exercise and bed-rest-mediated orthostatic intolerance. Med Sci Sports Exerc. 2002; 34: 1446-1453. PubMed: https://pubmed.ncbi.nlm.nih.gov/12218737/
- Schneider SM. Lee SM, Macias BR, Watenpaugh DE, Hargens AR. WISE-2005: exercise and nutrition countermeasures for upright VO2pk during bed rest. Med Sci Sports Exer. 2009; 41: 2165-2176. PubMed: https://pubmed.ncbi.nlm.nih.gov/19915502/
- Shibasaki M, Wilson TE, Bundgaard-Nielsen M, Seifert T, Secher NH, et al. Model flow underestimates cardiac output in heat-stressed individuals. Am J Physiol Regul Integr Comp Physiol. 2011; 300: R486–R491. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3043797/
- Summers RL, Platts S, Myers JG, Coleman TG. Theoretical analysis of the mechamisms of a gender differentiation in the propensity for orthostatic intolerance after spaceflight. Theoretical Biology and Medical Modeling. 2010; 7: 8.
- Sun XQ, Yao YJ, Wu XY, Jiang SZ, Jiang CL, et al. Effect of lower body negative pressure against orthostatic intolerance induced by 21 days head-down tilt bed rest. Aviat Space Environ Med. 2002; 73: 335-340. https://pubmed.ncbi.nlm.nih.gov/11952053/
- Watenpaugh DE, O’Leary DD, Schneider SM, Lee SM, Macias BR, et al. Lower body negative pressure exercise plus brief postexercise lower body negative pressure improve post-bed rest orthostatic tolerance. J Appl Physiol. 2007; 103: 1964-1972. PubMed: https://pubmed.ncbi.nlm.nih.gov/17947505/
- Wenner MM, Stachenfeld NS. Orthostatic Intolerance). In Morren FC (eds) Encyclopedia of Exercise Medicine in Health and Disease. Springer: Berlin, Heidelberg.2012; 665-667.
- Winker R, Barth A. Bidmon D, Ponocny I, Weber M, et al. Endurance exercise training in orthostatic intolerance: A randomized, controlled trial. Hypertension. 2005; 45: 391-398. PubMed: https://pubmed.ncbi.nlm.nih.gov/15699447/
- Zwart SR, Hargens AR, Lee SMC, Macias BR, Watenpaugh DE, et al. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone. 2007; 40: 529-537. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1876821/