More Information
Submitted: 04 November 2020 | Approved: 02 December 2020 | Published: 03 December 2020
How to cite this article: Adeagbo CA, Gbiri CAO, Olawale OA. Efficacy of transcranial direct current stimulation and over-ground walking task on functional mobility and quality of life of stroke survivors. J Nov Physiother Rehabil. 2020; 4: 049-056.
DOI:10.29328/journal.jnpr.1001037
Copyright License: © 2020 Adeagbo CA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Transcranial direct current stimulation; Over-ground walking; Lower limb; Quality of life; Stroke survivors
Efficacy of transcranial direct current stimulation and over-ground walking task on functional mobility and quality of life of stroke survivors
Caleb A Adeagbo*, Caleb AO Gbiri and Olajide A Olawale
Department of Physiotherapy, College of Medicine, University of Lagos, Lagos, Nigeria
*Address for Correspondence: Caleb Adewumi Adeagbo, Physiotherapy Department, College of Medicine, University of Lagos, Lagos, Nigeria, Tel: +234-8029308831; Email: [email protected]
Introduction: High proportion of stroke survivors have impaired functional mobility and decrease in overall quality of life (QoL). Transcranial direct current stimulation (tDCS) (non-invasive brain stimulation) and over-ground walking task (OGWT) (functional task-oriented training) have been suggested to improve functional mobility and QoL of stroke survivors. Hence, this study determined the efficacy of tDCS (anodal and cathodal) with OGWT on functional mobility and QoL of stroke survivors.
Materials and methods: Seventy eight (78) stroke survivors were randomised into three groups: anodal group (anodal tDCS with OGWT); cathodal group (cathodal tDCS with OGWT) and control group (OGWT only). Participants had two sessions of intervention per week for six weeks. Functional mobility was assessed using 10 meter walk test (10MWT) measuring steps, time and velocity while QoL was measured using Stroke Specific QoL (SSQoL) scale. Significance level was set at p < 0.05.
Results: Participants (46 males) were aged 56.78 ± 10.24 years. The groups were matched for functional mobility and QoL at baseline and only work/productivity domain of SSQoL showed statistically significant difference (p = 0.028). Each group showed statistically significant improvement between baseline and post-intervention scores of items in functional mobility (p ≤ 0.001) and total SSQoL (p ≤ 0.001). Anodal group showed better statistically significant improvement in step (p = 0.008), time (p = 0.024), velocity (p = 0.001) and total SSQoL (p = 0.016) among the groups when the mean differences were compared.
Conclusion: tDCS with OGWT is efficacious in improving functional mobility and QoL of stroke survivors. Specifically anodal tDCS with OGWT showed better clinical improvement in step, time, velocity and QoL in stroke survivors.
Stroke is a major health problem and it has been established to have wide-ranging effects on physical, mental, communication and social life of the survivor [1,2]. More than half of stroke survivors have impairment in motor, psychological and/or social functioning and these affect their quality of life (QoL) [3,4]. Motor functioning disorders in stroke include hemiplegia, hemiparesis, abnormal motor movement, balance abnormalities and all these negatively affect functional mobility and QoL especially in physical health, psychological health and the social functioning [4,5].
A high proportion of stroke survivors have impaired functional mobility and most of them walk with assistance from caregivers or using assistive devices such as walking stick or walking frame. Majority of stroke survivors who walk with assistive device or independently have reduced walking speed and increased number of steps and time to complete walking distance [6]. This therefore result to difficulty in performing activity of daily living, participating in community activities, reintegrating into the society and all these affect their global QoL.
Stroke survivors experience decrease in the overall QoL due to long-term impairments of motor and psychosocial functions from brain damage [7]. Quality of life is a personal perception of life in the context of culture and value system in which the person live and in relation to goals and expectations which is a complex process of interaction between personal traits, medical outcome, coping behaviour, social support and the quality of received health care [8]. The QoL of stroke is a comprehensive index of stroke recovery and comprises physical, psychological, emotional, and social aspects of recovery.
In the last few years, non-invasive brain stimulation (NIBS) modalities such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) have been increasingly used as adjunct therapy for motor impairment and psychosocial challenges in neurological disorders [9]. The tDCS has been suggested to be an effective NIBS technique and uses a weak direct electrical current that is applied to the scalp to modulate spontaneous neuronal firing in the human brain and it is painless [10].
Transcranial direct current stimulation induces neuroplasticity through generation of a subthreshold current, stimulating polarity-dependent alteration of membrane potentials and modifying spontaneous discharge rates [11,12]. Anodal tDCS stimulation induces neuronal membrane depolarization, cathodal tDCS stimulation induces neuronal hyperpolarization while anodal and cathodal tDCS stimulation applied simultaneously (bi-hemispheric stimulation) could hypothetically provide concurrent stimulation of both cortices [13,14].
Over the last few years several stroke rehabilitation approaches have been developed and their efficacy tested at promoting recovery after stroke [15]. Stroke rehabilitation programmes in which movement related to functional activities are directly trained (functional task-oriented programme) have shown better results than impairment-focused programmes [16]. Over-ground walking task (OGWT) is a form of functional task-oriented programme which is relatively cheap, can be performed in almost all environment and can be employed as therapeutic modality by rehabilitation expert and as home programme activity for stroke survivors to improve functional mobility [17]. This study was therefore aimed to determine efficacy of tDCS and OGWT on functional mobility and QoL of stroke survivors.
This study design was a single-blinded (patient-blinded), randomized controlled trial conducted at selected hospitals in Lagos State, Nigeria: the Lagos University Teaching Hospital (LUTH) Idi Araba; Lagos State University Teaching Hospital (LASUTH) Ikeja; General Hospital Gbagada; General Hospital Isolo and General Hospital Marina. The research protocol was approved by Health Research and Ethics Committees of the selected hospitals (ADM/DCST/HREC/APP/1740, LREC/06/10/908 and LSHSC/2222/VOL.VIA/219). The study was also registered with the South African Medical Research Council (Pan African Clinical Trial Registry) with unique identification registry number PACTR201809766183160.
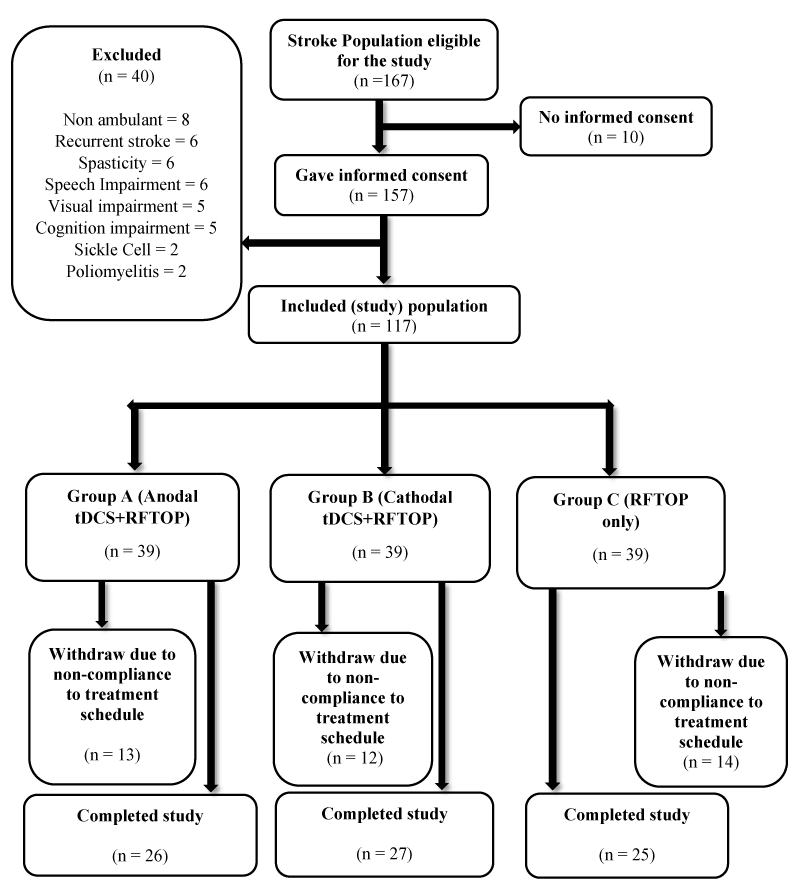
Inclusion criteria were First-ever stroke survivors older than eighteen (18) years with a diagnosis of stroke verified according to the definition by World Health Organisation, with not less than 3 months after stroke onset, able to understand verbal, graphic, pictorial and written instructions, a score more than or equal to 60 points on Barthel Index Scale and were presenting with hemiparesis but were able to ambulate independently with or without an ambulatory assistive device (cane). Participants were excluded if they were participating in another experimental study, with pre-existing neurological conditions (head injury, spinal cord injury, lower motor lesion and peripheral nerve lesion), with pre-existing psychiatric and psychological conditions (dementia, schizophrenia), with an intracranial metallic implant (cochlear implants, aneurysm clips and brain electrodes) or cardiac pacemaker (Figure 1).
Figure 1: Flow Chart of participants from recruitment to completion of study. n = number of participants.
The minimum sample size was calculated to be 19 participants per group, attrition and mortality rate was set at 20% [19]. Therefore, the estimated minimum sample size was n = 23 participants per group.
The baseline assessments were performed after written informed consent was obtained from all the participants. Participants were randomly assigned to anodal group, cathodal group and control group by asking participants to blindly select crushed pieces of paper with group name from a container. Participants were blinded to the stimulation type they received.
The Barthel Index scale was used to determine the level of activity of daily living and participants with less than 60 score were excluded. 10-metre walk test (10MWT) was used to assess functional mobility. A marked 14-meter walkway without obstruction was used and marked at 2-meter distance for acceleration, from the 2-meter to 12-meter for the actual 10-meter walk and from the 12-meter to the 14-meter for deceleration. Participants performed the task twice at natural pace and without external inducement from accessor to walk fast or slow. The average steps and time were calculated while velocity was calculated by dividing the distance covered (10-metre) by the average time taken. This assessment was performed at baseline and 6 weeks post-intervention.
Stroke-Specific Quality of Life (SSQoL) scale was used to assess the QoL of the participants. It is a patient-centred outcome measure intended to provide an assessment of health-related QoL specific to stroke survivors. The scale is a self-report scale containing 49 items in 12 domains: mobility, energy, upper extremity function, work/productivity, mood, self-care, social roles, family roles, vision, language, thinking, and personality. Each item was rated on a 5-point Likert scale. Higher scores indicate better function. Stroke-Specific Quality of Life scale yields both domain scores and an overall SSQoL scale summary score.
All participants were out-patients and had individualized therapy (i.e. participants were rehabilitated independently under closed supervision of trained physiotherapists). Participants in tDCS had 20 minutes of stimulation and 30 minutes of OGWT while control group had 30 minutes of OGWT only. All participants also had stretching, strengthening and range of motion exercises to the neck, trunk, upper and lower limbs. Participants were treated twice in a week for 6 weeks.
Participants in non-invasive brain stimulation group had tDCS current of 1.6mA for 20 minutes using a battery-driven, one channel stimulator manufactured by TCT Research Limited, Hong Kong with model number: M101 A-2012 and Serial number: !021!0M101A!00000BF7. Surface sponge electrodes (saline-soaked) with surface area of 25 cm2 (5 × 5 cm) for the active electrode and 35 cm2 (5 × 7 cm) for the dispersing electrode were used.
Anodal tDCS electrode placement was performed by placing the anode electrode on the primary motor cortex (M1) i.e. on C3/C4 of the affected hemisphere and the dispersing electrode on the contralateral supraorbital area while cathodal tDCS electrode placement was performed by placing the cathode electrode on the C3/C4 of the unaffected hemisphere and the dispersing electrode on the contralateral supraorbital area.
All participants were rehabilitated with over-ground walking. The over ground training involved participants walking on the gym floor at participants own pace for about a minute then the intensity of the walking was increased by increasing the pace during walking for about 3 minutes and slowly back to normal walking pace for about a minute. The OGWT was performed three times per session after 5 minutes rest.
Data were analysed using the Statistical Package for Social Sciences (SPSS) version 23.0. Wilcoxon signed-rank test and paired t-test were used for comparisons within groups for non-parametric and parametric variables respectively. Kruskal-Wallis and One way Analysis of Variance (ANOVA) was used for comparisons of non-parametric and parametric variables respectively among the 3 groups. Post Hoc analysis was used to determine the group changes. The level of significance was set at p < 0.05.
Participants’ age ranged between 38 years to 86 years and the mean age was 56.78 ± 10.24 years. Forty-six (59.0%) participants were male while 32 (41.0%) were female. Forty-four (56.4%) participants had left hemispheric stroke while 34 (43.6%) had right hemispheric stroke. There were 26 participants in anodal group, 27 participants in cathodal group and 25 participants in control group.
Changes were observed when the baseline mean scores of items in functional mobility were compared with the post-intervention mean scores after 6 weeks intervention for the anodal, cathodal and control groups (Table 1). Number of steps decreased from 25.77 ± 11.69 to 20.46 ± 10.01 in the anodal group, from 26.41 ± 10.58 to 22.96 ± 9.42 in cathodal group and from 23.88 ± 9.40 to 21.04 ± 7.68 in control group (Table 1).
| Table 1: Comparison of functional mobility items within the groups using paired t - test. | |||||
| Baseline ± SD | Post-Intervention ± SD | t - value | p - value | ||
| 10 MWT ITEMS | |||||
| Anodal (n = 26) | Steps | 25.77 ± 11.69 | 20.46 ± 10.01 | 7.642 | < 0.001* |
| Time (seconds) | 22.18 ± 17.86 | 16.82 ± 13.72 | 4.454 | < 0.001* | |
| Velocity (m/s) | 0.63 ± 0.27 | 0.81 ± 0.32 | -7.755 | < 0.001* | |
| Cathodal (n = 27) | Steps | 26.41 ± 10.58 | 22.96 ± 9.42 | 7.2 | < 0.001* |
| Time (seconds) | 24.62 ± 20.28 | 21.20 ± 17.88 | 4.703 | < 0.001* | |
| Velocity (m/s) | 0.54 ± 0.23 | 0.63 ± 0.26 | -5.191 | < 0.001* | |
| Control (n = 25) | Steps | 23.88 ± 9.40 | 21.04 ± 7.68 | 5.654 | < 0.001* |
| Time (seconds) | 18.90 ± 12.08 | 16.91 ± 11.05 | 5.608 | < 0.001* | |
| Velocity (m/s) | 0.69 ± 0.32 | 0.78 ± 0.36 | -3.706 | 0.001* | |
| * Significant at p < 0.05. | |||||
Time also reduced from 22.18 ± 17.86 to 16.82 ± 13.72, 24.62 ± 20.28 to 21.20 ± 17.88 and 18.90 ± 12.08 to 16.91 ± 11.05 for the anodal, cathodal and control groups respectively (Table 1). The velocity increased from 0.63 ± 0.27 to 0.81 ± 0.32, 0.54 ± 0.23 to 0.63 ± 0.26 and 0.69 ± 0.32 to 0.78 ± 0.36 for the anodal, cathodal and control groups respectively (Table 1).
Within group comparison of step, time and velocity showed that there was statistical significant difference p ≤ 0.001 for the anodal, cathodal and control groups (Table 1).
However, there was no statistical significant difference (p ≥ 0.05) among the mean scores of functional mobility items when the 3 groups were matched at baseline and after 6 weeks intervention (Table 2). Comparison of mean difference of items in functional mobility showed statistical difference in step (p = 0.008), time (p = 0.024) and velocity (0.001) (Table 3).
| Table 2: Comparison of functional mobility among the groups using One-Way ANOVA. | ||||||
| Anodal (n = 26) ± SD | Cathodal (n = 27) ± SD | Control (n = 25) ± SD | f - value | p - value | ||
| 10 MWT ITEMS | ||||||
| Baseline | Steps | 25.77 ± 11.69 | 26.41 ± 10.58 | 23.88 ± 9.40 | 0.394 | 0.676 |
| Time | 22.18 ± 17.86 | 24.62 ± 20.28 | 18.90 ± 12.08 | 0.72 | 0.49 | |
| Velocity | 0.63 ± 0.27 | 0.54 ± 0.23 | 0.69 ± 0.32 | 1.909 | 0.155 | |
| Post-Intervention | Steps | 20.46 ± 10.01 | 22.96 ± 9.42 | 21.04 ± 7.68 | 0.549 | 0.58 |
| Time | 16.82 ± 13.72 | 21.20 ± 17.88 | 16.91 ± 11.05 | 0.778 | 0.463 | |
| Velocity | 0.81 ± 0.31 | 0.63 ± 0.26 | 0.78 ± 0.36 | 2.482 | 0.09 | |
| Table 3: Comparison of functional mobility among the groups using One-Way ANOVA. | ||||||
| (a) Anodal (n = 26) MD ± SD |
(b) Cathodal (n = 27) MD ± SD |
(c) Control (n = 25) MD ± SD |
f - value | p - value | Post Hoc | |
| 10 MWT ITEMS | ||||||
| Steps | -5.31 ± 3.54 | 3.44 ± 2.49 | -2.83 ± 2.51 | 5.105 | 0.008* | a&c |
| Time (seconds) | -5.35 ± 6.13 | -3.42 ± 3.78 | -1.99 ± 1.77 | 3.943 | 0.024* | a&c |
| Velocity (m/s) | 0.18 ± 0.12 | 0.09 ± 0.08 | 0.08 ± 0.12 | 7.236 | 0.001* | a&b, a&c |
| * Significant at p < 0.05. | ||||||
The significant change was seen between the anodal and control group in step and time while the significant change in velocity was between anodal and cathodal group and also anodal and control group (Table 3).
Increase were observed when the baseline mean scores of all the domains of SSQoL were compared with the post-intervention mean scores after 6 weeks intervention for the anodal, cathodal and control groups (Table 4). The total anodal group mean scores at baseline and post-intervention were 196.73 ± 28.59 and 211.04 ± 25.01. The total cathodal group mean scores at baseline and post-intervention were 201.63 ± 26.93 and 206.85 ± 23.12 while total control group mean scores at baseline and post-intervention were 207.32 ± 23.37 and 212.32 ± 23.07 (Table 4). There was significant difference (p < 0.05) in energy, family roles, mobility, mood, personality, self-care, social roles, thinking, upper extremity function, work/productivity domains and total SSQoL score of the anodal group (Table 4). There was significant difference (p < 0.05) in energy, family roles, mobility, self-care, social roles, upper extremity function domains and total SSQoL score of the cathodal group (Table 4) while there was significant difference (p < 0.05) in energy, family roles, mobility, social roles, thinking domains and total SSQoL score of the control group (Table 4).
| Table 4: Comparison of stroke specific quality of life within the groups using Wilcoxon Signed Rank Test. | |||||
| Stroke specific quality of life | Baseline ± SD | Post-Intervention ± SD | u - value | z - value | p - value |
| ANODAL (n = 26) | |||||
| Energy | 10.88 ± 3.47 | 12.58 ± 2.98 | 66 | -2.95 | 0.003* |
| Family roles | 11.62 ± 2.53 | 12.81 ± 2.28 | 66 | -2.956 | 0.003* |
| Language | 23.27 ± 4.23 | 23.85 ± 2.94 | 6 | -1.732 | 0.083 |
| Mobility | 25.27 ± 5.85 | 27.15 ± 4.61 | 45 | -2.673 | 0.008* |
| Mood | 20.08 ± 4.73 | 21.38 ± 3.82 | 36 | -2.527 | 0.012* |
| Personality | 11.77 ± 3.05 | 12.73 ± 2.59 | 28 | -2.371 | 0.018* |
| Self-care | 20.73 ± 3.57 | 22.00 ± 3.05 | 55 | -2.829 | 0.005* |
| Social roles | 15.58 ± 5.76 | 17.54 ± 5.54 | 78 | -3.066 | 0.002* |
| Thinking | 12.12 ± 2.23 | 12.85 ± 2.38 | 21 | -2.214 | 0.027* |
| Upper extremity function | 19.19 ± 5.73 | 21.00 ± 4.53 | 78 | -3.089 | 0.002* |
| Vision | 14.38 ± 1.30 | 14.58 ± 1.03 | 3 | -1.342 | 0.18 |
| Work/Productivity | 11.85 ± 3.20 | 12.58 ± 2.72 | 21 | -2.232 | 0.026* |
| QoL Total | 196.73 ± 28.59 | 211.04 ± 25.01 | 253 | -4.109 | <0.001* |
| CATHODAL (n = 27) | |||||
| Energy | 11.70 ± 2.30 | 12.52 ± 1.87 | 36 | -2.546 | 0.011* |
| Family roles | 11.67 ± 2.94 | 12.26 ± 2.54 | 28 | -2.379 | 0.017* |
| Language | 23.67 ± 3.57 | 23.70 ± 3.28 | 3 | 0 | 1 |
| Mobility | 24.96 ± 5.55 | 26.19 ± 4.81 | 45 | -2.673 | 0.008* |
| Mood | 20.85 ± 3.28 | 21.00 ± 3.06 | 6 | -1.633 | 0.102 |
| Personality | 12.48 ± 2.10 | 12.52 ± 2.05 | 1 | -1 | 0.317 |
| Self-care | 20.44 ± 2.90 | 20.85 ± 2.91 | 21 | -2.232 | 0.026* |
| Social roles | 16.30 ± 5.67 | 17.70 ± 4.32 | 64 | -2.769 | 0.006* |
| Thinking | 12.52 ± 2.36 | 12.59 ± 2.31 | 1 | -1 | 0.317 |
| Upper extremity function | 20.81 ± 3.85 | 21.15 ± 3.42 | 21 | -2.251 | 0.024* |
| Vision | 14.33 ± 1.27 | 14.33 ± 1.27 | 0 | 0 | 1 |
| Work/Productivity | 11.89 ± 3.17 | 12.04 ± 2.59 | 4.5 | -0.816 | 0.414 |
| QoL Total | 201.63 ± 26.93 | 206.85 ± 23.12 | 178 | -3.35 | 0.001* |
| CONTROL (n = 25) | |||||
| Energy | 12.08 ± 2.43 | 12.84 ± 2.10 | 36 | -2.565 | 0.010* |
| Family roles | 12.40 ± 2.94 | 13.04 ± 2.75 | 21 | -2.333 | 0.020* |
| Language | 23.16 ± 3.41 | 23.24 ± 3.24 | 2 | -0.447 | 0.655 |
| Mobility | 25.76 ± 4.83 | 26.72 ± 4.55 | 28 | -2.375 | 0.018* |
| Mood | 20.84 ± 3.88 | 21.24 ± 3.77 | 10 | -1.841 | 0.066 |
| Personality | 12.80 ± 3.99 | 13.12 ± 3.23 | 34 | -1.373 | 0.17 |
| Self-care | 20.92 ± 3.28 | 21.48 ± 3.66 | 13 | -1.49 | 0.136 |
| Social roles | 17.00 ± 4.51 | 17.84 ± 4.53 | 53.5 | -2.714 | 0.007* |
| Thinking | 13.44 ± 1.53 | 13.56 ± 1.53 | 1 | -1 | 0.317 |
| Upper extremity function | 20.96 ± 3.25 | 21.24 ± 3.24 | 16 | -1.99 | 0.049* |
| Vision | 14.24 ± 1.30 | 14.28 ± 1.31 | 1 | -1 | 0.317 |
| Work/Productivity | 13.72 ± 1.65 | 13.72 ± 1.65 | 0 | 0 | 1 |
| QoL Total | 207.32 ± 23.37 | 212.32 ± 23.07 | 188.5 | -3.768 | < 0.001* |
| * Significant at p < 0.05. | |||||
Among group comparison showed statistically significant difference at baseline for Work/Productivity domain (p = 0.028) and post-intervention Work/Productivity domain (p = 0.042) (Table 5). Post Hoc analysis revealed that the significant difference was between anodal and control and also cathodal and control at baseline while between the cathodal and control at post-intervention (Table 5).
| Table 5: Comparison of SSQOL among the groups using Kruskal-Wallis Test. | |||||||
| Stroke specific quality of life | (a) Anodal (n = 26) ± SD | (b) Cathodal (n = 27) ± SD | (c) Control (n = 25) ± SD | k - value | p - value | Post Hoc | |
| Baseline | Energy | 10.88 ± 3.47 | 11.70 ± 2.30 | 12.08 ± 2.43 | 1.387 | 0.5 | |
| Family roles | 11.62 ± 2.53 | 11.67 ± 2.94 | 12.40 ± 2.94 | 2.109 | 0.348 | ||
| Language | 23.27 ± 4.23 | 23.67 ± 3.57 | 23.16 ± 3.41 | 0.951 | 0.622 | ||
| Mobility | 25.27 ± 5.85 | 24.96 ± 5.55 | 25.76 ± 4.83 | 0.363 | 0.834 | ||
| Mood | 20.08 ± 4.73 | 20.85 ± 3.28 | 20.84 ± 3.88 | 0.094 | 0.954 | ||
| Personality | 11.77 ± 3.05 | 12.48 ± 2.10 | 12.80 ± 3.99 | 0.441 | 0.802 | ||
| Self-care | 20.73 ± 3.57 | 20.44 ± 2.90 | 20.92 ± 3.28 | 0.319 | 0.853 | ||
| Social roles | 15.58 ± 5.76 | 16.30 ± 5.67 | 17.00 ± 4.51 | 0.605 | 0.739 | ||
| Thinking | 12.12 ± 2.23 | 12.52 ± 2.36 | 13.44 ± 1.53 | 4.706 | 0.095 | ||
| Upper extremity function | |||||||
| Vision | 19.19 ± 5.73 | 20.81 ± 3.85 | 20.96 ± 3.25 | 0.911 | 0.634 | ||
| Work/Productivity | 14.38 ± 1.30 | 14.33 ± 1.27 | 14.24 ± 1.30 | 0.367 | 0.832 | ||
| QoL Total | 11.85 ± 3.20 | 11.89 ± 3.17 | 13.72 ± 1.65 | 7.177 | 0.028* | a&c, b&c | |
| 196.73 ± 28.59 | 201.63 ± 26.93 | 207.32 ± 23.37 | 2.086 | 0.352 | |||
| Post-Intervention | Energy | ||||||
| Family roles | 12.58 ± 2.98 | 12.52 ± 1.87 | 12.84 ± 2.10 | 1.192 | 0.551 | ||
| Language | 12.81 ± 2.28 | 12.26 ± 2.54 | 13.04 ± 2.75 | 1.947 | 0.378 | ||
| Mobility | 23.85 ± 2.94 | 23.70 ± 3.28 | 23.24 ± 3.24 | 1.197 | 0.55 | ||
| Mood | 27.15 ± 4.61 | 26.19 ± 4.81 | 26.72 ± 4.55 | 0.808 | 0.668 | ||
| Personality | 21.38 ± 3.82 | 21.00 ± 3.06 | 21.24 ± 3.77 | 0.592 | 0.744 | ||
| Self-care | 12.73 ± 2.59 | 12.52 ± 2.05 | 13.12 ± 3.23 | 0.583 | 0.747 | ||
| Social roles | 22.00 ± 3.05 | 20.85 ± 2.91 | 21.48 ± 3.66 | 2.05 | 0.359 | ||
| Thinking | 17.54 ± 5.54 | 17.70 ± 4.32 | 17.84 ± 4.53 | 0.336 | 0.845 | ||
| Upper extremity function | 12.85 ± 2.38 | 12.59 ± .31 | 13.56 ± 1.53 | 2.319 | 0.314 | ||
| Vision | |||||||
| Work/Productivity | 21.00 ± 4.53 | 21.15 ± 3.42 | 21.24 ± 3.24 | 0.05 | 0.975 | ||
| QoL Total | 14.58 ± 1.03 | 14.33 ± 1.27 | 14.28 ± 1.31 | 0.796 | 0.672 | ||
| 12.58 ± 2.72 | 12.04 ± 2.59 | 13.72 ± 1.65 | 6.323 | 0.042* | b&c | ||
| 211.04 ± 25.01 | 206.85 ± 23.12 | 212.32 ± 23.07 | 0.793 | 0.673 | |||
| *Significant at p < 0.05. | |||||||
Statistically significant difference was noted in the mean difference among the groups at the thinking (p = 0.029), upper extremity function (p = 0.014) and work/productivity (0.028) domains and total SSQoL (p = 0.016) (Table 6). Post hoc analysis showed the significant differences were between anodal and cathodal, anodal and control for thinking, upper extremity function and total SSQoL while it was anodal and control for work/productivity (Table 6).
| Table 6: Comparison of stroke specific quality of life among the groups using Kruskal-Wallis Test. | ||||||
| Stroke specific quality of life | (a) Anodal (n = 26) MD ± SD | (b) Cathodal (n = 27) MD ± SD | (c) Control (n = 25) MD ± SD | k - value | p - value | Post Hoc |
| Energy | 1.69 ± 2.43 | 0.82 ± 1.50 | 0.76 ± 1.20 | 2.248 | 0.325 | |
| Family roles | 1.19 ± 1.72 | 0.59 ± 1.15 | 0.64 ± 1.22 | 2.683 | 0.261 | |
| Language | 0.58 ± 1.63 | 0.04 ± 1.32 | 0.08 ± 1.19 | 1.839 | 0.399 | |
| Mobility | 1.89 ± 3.33 | 1.22 ± 2.03 | 0.96 ± 1.93 | 0.595 | 0.743 | |
| Mood | 1.31 ± 2.56 | 0.15 ± 0.46 | 0.40 ± 1.16 | 4.284 | 0.117 | |
| Personality | 0.96 ± 1.89 | 0.04 ± 0.19 | 0.32 ± 1.52 | 4.798 | 0.091 | |
| Self-care | 1.27 ± 1.95 | 0.41 ± 0.84 | 0.56 ± 2.12 | 4.2 | 0.122 | |
| Social roles | 1.96 ± 2.99 | 1.41 ± 2.28 | 0.84 ± 1.43 | 1.532 | 0.465 | |
| Thinking | 0.73 ± 1.46 | 0.07 ± 0.39 | 0.12 ± 0.60 | 7.068 | 0.029* | a&b, a&c |
| Upper extremity function | ||||||
| Vision | 1.81 ± 2.61 | 0.33 ± 0.68 | 0.28 ± 0.68 | 8.567 | 0.014* | a&b, a&c |
| Work/Productivity | 0.19 ± 0.69 | 0.00 ± 0.00 | 0.40 ± 0.20 | 2.145 | 0.342 | |
| QoL Total | 0.73 ± 1.51 | 1.15 ± 1.13 | 0.00 ± 0.00 | 7.128 | 0.028* | a&c |
| 14.31 ± 14.22 | 5.22 ± 6.65 | 5.00 ± 6.31 | 8.312 | 0.016* | a&b, a&c | |
| * Significant at p < 0.05. | ||||||
This study was conducted to determine the effects of tDCS and OGWT on functional mobility and QoL of stroke survivors. The effects of the therapies on functional mobility were monitored by 10MWT measuring number of steps, time and velocity while QoL was measured by SSQoL at baseline and 6 week post intervention. The interventions were anodal tDCS with OGWT for anodal group, cathodal tDCS with OGWT for cathodal group and OGWT only for control group. The outcome of this study showed that the 6-week intervention resulted into clinical improvement in number of steps, time, velocity and SSQoL scores. However, participants in anodal group had higher significant gain in all the items measured during 10MWT and domains of QoL. The major finding of this study was that anodal tDCS with OGWT results to greater clinical improvement in mobility function and QoL than cathodal tDCS with OGWT and OGWT alone.
Transcranial direct current stimulation has been shown to improve force generation, motor control and joint coordination in lower limb leading to improve functional mobility while OGWT has also been shown to promote functional mobility among stroke survivors [17,18,20,21]. An, et al. [7] in a study titled effect of tDCS of stroke patients on depression and quality of life reported improvement in QoL of stroke survivors. Nevertheless there is no published study on effects of tDCS and OGWT on functional mobility and QoL.
The main effect of tDCS on the brain is modulation of the resting membrane potentials, tDCS can cause either neuronal membrane depolarization (long term potentiation) or hyperpolarization (long term depression) depending on the type of electrode placement resulting in different type of stimulation while the main effect of OGWT on the brain and lower limb is motor learning, control and skill which translate to functional mobility and improve QoL [17-21].
Stroke rehabilitation requires more than 5 sessions of therapy to detect clinical and statistical changes in outcome measures. The effects of tDCS is reversible when the number of sessions are short e.g. less than 5 treatment sessions and duration of treatment per session also short (less than 10 minute stimulation per session). Smaller tDCS electrode size, higher charge density and higher current density are also associated with greater improvement in motor recovery in stroke rehabilitation [22]. Stroke rehabilitation programmes incorporating functional activities show better motor recovery and translate to functional performance of activity of daily living [16] and OGWT is a functional task-oriented programme.
The outcome of this study showed that scores from items measured during 10MWT and domains of SSQoL among the 3 groups showed no significant difference at baseline except work/productivity and also at post-intervention indicating no statistically significant superiority of the interventions used for the groups. However mean difference among group showed clinical and statistically significant difference in all items measured during 10MWT, thinking, upper extremity function, work/productivity and total QoL. Anodal tDCS with OGWT showed better statistically improved mean difference compared to cathodal tDCS with OGWT and OGWT alone in functional mobility and QoL. This outcome may be due to depolarization of the neuronal membrane resulting to excitation in the brain causing increase synaptic ability and changes in neurotransmitters.
The outcome of this study also showed that the different therapies (anodal+OGWT, cathodal+OGWT and OGWT) clinically increased mobility functions in stroke survivors and improved QoL. Participants made significant decrease in number of steps and amount of time to complete a walking distance. Also there were improvements in energy, family roles, mobility, mood, personality, self-care, social roles, thinking, upper extremity function, work/productivity and total QoL. No much changes were seen in language and vision domain of SSQoL and this may be due to the fact that most of the participants were not having challenges in language and vision.
Improvements recorded in the anodal group i.e. participants treated with anodal tDCS and OGWT were similar with the findings of previous study by Geroin, et al. [23] who reported a statistical significant improvement in 10MWT after anodal stimulation and robotic gait training in chronic stroke. This also agreed with feasibility study by Danzl, et al. [24] who reported greater improvement in the anodal tDCS in study population that had robotic gait orthosis. Manji, et al, [25] also corroborated this report in a study where participants had Body Weight Support and Treadmill Training and anodal tDCS group improved in gait speed and applicative walking. The improvement in this study was due to depolarization of the brain by anodal tDCS and functional repetitive practice of mobility by participants.
The improvement recorded in the cathodal group i.e. cathodal tDCS and OGWT was in agreement with a previous study on cathodal tDCS as adjunct therapy in improving motor functioning at activity level for lower limb in stroke survivors [26]. This improvement may be due to hyperpolarization of the contralessional hemisphere of the brain by cathodal tDCS and functional task training by OGWT.
Overground walking training has been shown in the literature to improve lower limb functioning at the activity level (functional mobility) [17,18,20,25,27-29]. It is a repetitive functional task practice and the improvement seen in participants that received only OGWT can be attributed to neuroplasticity in the brain and motor control and skill development in the lower limb after repeated practice of functional tasks that are challenging to the ability of the stroke survivors. Therefore the improvement in the functional mobility lead to improvement in QoL.
Based on the findings of this study, it was concluded that stimulation of the brain with tDCS is safe and also efficacious as an adjunct therapy. Anodal stimulation is a better electrode placement for tDCS in improving functional mobility and QoL. Overground walking task is effective therapy to improve functional mobility.
The authors would like to thank the following participating centres and research assistants for their contributions to the success of this research.
Participating centres: Lagos University Teaching Hospital (LUTH) Idi-Araba; Lagos State University Teaching Hospital (LASUTH) Ikeja; General Hospital Gbagada; General Hospital, Isolo and General Hospital Marina.
Research assistants: Mr. Daniel Ayeni and Miss. Sola Sholarin. They assisted in patient assessment at baseline and post-intervention in the approach described in the methodology.
Disclosures
The authors declare that there is no conflict of interest. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
- Chen C, Tsai C, Chung C, Chen C, Wu KPH, et al. Potential predictors for health-related quality of life in stroke patients undergoing inpatient rehabilitation. Health Qual Life Out. 2015; 13: 1-10.
- De Wit L, Theuns P, Dejaeger E, Devos S, Gantenbein AR, et al. Long-term impact of stroke on patients’ health-related quality of life. Disabil. Rehabil. 2017; 39: 1435-1440 PubMed: https://pubmed.ncbi.nlm.nih.gov/27385479/
- Walsh ME, Galvin R, Loughnane C, Macey C, Horgan NF. Community re-integration and long-term need in the first five years after stroke: results from a national survey. Disabil Rehabil. 2015; 37: 1834-1838. PubMed: https://pubmed.ncbi.nlm.nih.gov/25391817/
- Badaru UM, Ogwumike OO, Adeniyi AF. Health related quality of life of stroke survivors in Africa: a critical review of literature. Arch Physiother Glob Res. 2015; 19: 7-16.
- Alghwiri AA. The Correlation between Depression, Balance and Physical Functioning Post Stroke. J Stroke Cerebrovasc. 2016; 25: 475-479. PubMed: https://pubmed.ncbi.nlm.nih.gov/26617326/
- Mehrholz J, Pohl M, Kugler J, Elsner B. The Improvement of Walking Ability Following Stroke a Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Dtsch Arztebl Int. 2018; 115: 639–645. PubMed: https://pubmed.ncbi.nlm.nih.gov/30375325/
- An T, Kim S, Kim K. Effect of transcranial direct current stimulation of stroke patients on depression and quality of life. J Phys Ther Sci. 2017; 29: 505-507. PubMed: https://pubmed.ncbi.nlm.nih.gov/28356641/
- Gbiri CA, Akinpelu AO. Quality of life of Nigerian stroke survivors during first 12 months post-stroke. Hong Kong Physiother J. 2012; 30: 18-24.
- Mortensen J, Figlewski K, Andersen H. Combined transcranial direct current stimulation and home-based occupational therapy for upper limb motor impairment following intracerebral hemorrhage: a double-blind randomized controlled trial. Disabil Rehabil. 2016; 38: 637-643. PubMed: https://pubmed.ncbi.nlm.nih.gov/26079636/
- Rocha S, Silva E, Foerster A, Wiesiolek C, Chagas AP, et al. The impact of transcranial direct current stimulation (tDCS) combined with modified constraint induced movement therapy (mCIMT) on upper limb function in chronic stroke: a double blind randomized controlled trial. Disabil. Rehabil. 2016; 38: 653-660. PubMed: https://pubmed.ncbi.nlm.nih.gov/26061222/
- Eryilmaz G, Sayar GH, Ünsalver BO, Gül IG, Özten E, et al. Adverse Effects of Transcranial Direct Current Stimulation (TDCS) in a Group of Psychiatric Patients. SJAMS. 2014; 2: 294-297.
- Kuo M, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage. 2014; 85: 948-960. PubMed: https://pubmed.ncbi.nlm.nih.gov/23747962/
- Fusco A, De Angelis D, Morone G, Maglione L, Paolucci T, et al. The ABC of tDCS: Effects of Anodal, Bilateral and Cathodal Montages of Transcranial Direct Current Stimulation in Patients with Stroke - A Pilot Study. Stroke Res Treat. 2013; 2013: 837595. PubMed: https://pubmed.ncbi.nlm.nih.gov/23365790/
- Fleming MK, Rothwell JC, Sztriha L, Teo JT, Newhama DJ. The effect of transcranial direct current stimulation on motor sequence learning and upper limb function after stroke. Clin Neurophysiol. 2017; 128: 1389-1398. PubMed: https://pubmed.ncbi.nlm.nih.gov/28410884/
- Claflin ES, Krishnan C, Khot SP. Emerging treatments for motor rehabilitation after stroke. The Neurohospitalist. 2015; 5: 77-88. PubMed: https://pubmed.ncbi.nlm.nih.gov/25829989/
- Pollock A, Baer G, Campbell P, Choo PL, Forster A, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke (Review). In: Cochrane database of systematic reviews (Fourth Edition). John Wiley & Sons Ltd. 2014; 1-433. PubMed: https://pubmed.ncbi.nlm.nih.gov/24756870/
- Olawale OA, Jaja SI, Anigbogu CN, Appiah-Kubi KO, Jones-Okai D. Exercise training improves walking function in an African group of stroke survivors: a randomized controlled trial. Clin Rehabil. 2011; 25: 442-450. PubMed: https://pubmed.ncbi.nlm.nih.gov/21427155/
- Badaru UM, Ja’afar M, Hassan Isa UL, Rufai YA. Comparative efficacy of treadmill training and combination of bicycle ergometer and over-ground walk on functional ambulation post-stroke - a pilot study. Fizjoterapia. 2019; 86-94.
- Bornheim S, Croisier J, Maquet P, et al. Brain Stimulation Transcranial direct current stimulation associated with physical- therapy in acute stroke patients - A randomized, triple blind, sham-controlled study. Brain Stimul. 2020; 13: 329-336. PubMed: https://pubmed.ncbi.nlm.nih.gov/31735645/
- van Asseldonk EHF. Transcranial Direct Current Stimulation Enhances Propulsion during Walking. W. Jensen et al. (eds.). Replace, Repair, Restore, Relieve – Bridging Clinical and Engineering Solutions in Neurorehabilitation, Biosystems & Biorobotics. 2014; 7: 805.
- Bai X, Guo Z, He L, Ren L, McClure MA, et al.Different Therapeutic Effects of Transcranial Direct Current Stimulation on Upper and Lower Limb Recovery of Stroke Patients with Motor Dysfunction: A Meta-Analysis. Neural Plast. 2019; 2019: 1372138. PubMed: https://pubmed.ncbi.nlm.nih.gov/31827495/
- Chhatbar PY, Ramakrishnan V, Kautz S, George MS, Adams RJ, et al. Transcranial Direct Current Stimulation post-stroke upper extremity motor recovery studies exhibit a dose-response relationship. Brain Stimul. 2016; 9: 16-26. PubMed: https://pubmed.ncbi.nlm.nih.gov/26433609/
- Geroin C, Picelli A, Munari D, Waldner A, Tomelleri C, et al. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: a preliminary comparison. Clin Rehabil 2011; 25: 537-548. PubMed: https://pubmed.ncbi.nlm.nih.gov/21402651/
- Danzl MM, Chelette KC, Lee K, Lykins D, Sawaki L. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. NeuroRehabilitation 2013; 33: 67-76. PubMed: https://pubmed.ncbi.nlm.nih.gov/23949035/
- Manji A, Amimoto K, Matsuda T, Wada Y, Inaba A, et al. Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neuroscience Letter. 2017; 662: 302-305. PubMed: https://pubmed.ncbi.nlm.nih.gov/29107706/
- Fusco A, Assenza F, Iosa M, Altavilla R, Paolucci S, et al. The ineffective role of cathodal tDCS in enhancing the functional motor outcomes in early phase of stroke rehabilitation: an experimental trial. Biomed Res Int. 2014; 2014: 547290. PubMed: https://pubmed.ncbi.nlm.nih.gov/24895588/
- Peurala SH, Tarkka IM, Pitkanen K, Sivenius J. The effectiveness of body weight-supported gait training and floor walking in patients with chronic stroke. Arch Phys Med Rehabil. 2005; 86: 1557-1564. PubMed: https://pubmed.ncbi.nlm.nih.gov/16084808/
- Park HJ, Oh DW, Kim SY, Choi JD. Effectiveness of community-based ambulation training for walking function of post-stroke hemiparesis: a randomized controlled pilot trial. Clin Rehabil. 2011; 25: 451-459. PubMed: https://pubmed.ncbi.nlm.nih.gov/21245205/
- Kim M, Cho K, Lee W. Community walking training program improves walking function and social participation in chronic stroke patients. Tohoku J Exp Med. 2014; 234: 281-286. PubMed: https://pubmed.ncbi.nlm.nih.gov/25483170/