More Information
Submitted: March 16, 2023 | Approved: March 23, 2022 | Published: March 24, 2023
How to cite this article: Vaher I, Tamm AL, Salus M, Reisberg K, Vähi A, et al. Effectiveness of massage chair and classic massage in recovery from physical exertion: a pilot study. J Nov Physiother Rehabil. 2023; 7: 008-015.
DOI: 10.29328/journal.jnpr.1001050
Copyright License: © 2023 Vaher I, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Effectiveness of massage chair and classic massage in recovery from physical exertion: a pilot study
Ivi Vaher1, Anna-Liisa Tamm1*, Marit Salus1, Kirkke Reisberg1, Aleksandra Vähi2, Helena Pallon1, Andra Paeste1, Bäthel-Betty Pirk2, Margus Merila2 and Thomas Schrader3
1Physiotherapy and Environmental Health Department, Tartu Health Care College, Tartu, Estonia
2Vocational Education Department, Tartu Health Care College, Tartu, Estonia
3Technische Hochschule Brandenburg, University of Applied Sciences, Department of Informatics and Media, Brandenburg, Germany
*Address for Correspondence: Anna-Liisa Tamm, Physiotherapy and Environmental Health Department, Tartu Health Care College, Tartu, Estonia, Email: [email protected]
Quick and cost-effective recovery is foundational to high-quality training and good competition results in today’s sports.
The aim of the research was to elucidate the effects of hand and massage chair massage on the biomechanical parameters of muscles of lower limbs and back, indicators of Pain Pressure Thresholds (PPT) and subjectively perceived fatigue.
A total of 32 female recreational athletes (18 – 50 years old) were assigned to a hand massage, massage chair, or lying down the group. They were measured for muscle biomechanical properties (MyotonPro), PPT (Wagner Instruments) and subjectively perceived fatigue (VAS scale) before and after fatigue tests and treatment. The recovery procedure and subjective satisfaction with treatment were rated on a Likert scale.
Changes in the median value of m. rectus femoris and m. gastrocnemius stiffness with treatment showed that hand massage could be more effective in reducing stiffness, as compared to chair massage.
Hand massage may have benefits for recovery from physical exertion, but due to the individuality of subjects, detailed methodological studies are needed to evaluate the effects of massage chair vs. hand massage.
At the training frequency of top athletes, a short recovery period between two workouts may not be enough to achieve the perfect readiness of muscles for new training [1]. Therefore, the choice of recovery techniques is crucial if an athlete is to participate in each subsequent workout rested, healthy and without injury [2]. Muscle stiffness—whether overly stiff or not stiff enough—leads to muscle damage [3]. There has not been a sufficient study of the effects of exercise-induced fatigue on muscle tone and stiffness and the effects of various possible recovery techniques on muscles [4]. Excessive muscle tone and stiffness result in a sharp increase in intramuscular vascular resistance, which reduces the amount of blood passing through the vessel per unit of time. In such a case, blood circulates at a normal volume rate between two consecutive contractions. At higher values of muscle stiffness, the decrease in intramuscular pressure is much slower and before the intra-muscular pressure reaches normal, a new contraction may begin. This disrupts the supply of oxygen to the muscle, resulting in earlier muscle fatigue. The muscle quickly recovers from the post-exertional state of tension if the muscle has good elasticity [5].
Several types of recovery techniques have been proposed to improve recovery after exercise, including pressure techniques such as massage [6], compression garments [7], water procedures [8], electrical stimulation [9], stretching [10], etc. Massage therapy is one of the most widely used therapeutic interventions. It affects both the structure and function of the muscular system and is effective in reducing muscle stiffness and perceived fatigue [11]. Massage seems to be the most effective method to reduce delayed-onset muscle pain and perceived fatigue, regardless of the person (i.e., athlete/non-athlete) [7]. It has been shown that a 20 minute - 30 minute massage performed immediately or up to 24 hours after training effectively reduces later muscle pain [6]. Likewise, a significant decrease in later muscle pain after a massage procedure has been observed in ultramarathon runners [12].
Mechanical massage refers to the manipulation of soft tissues by machines, including a Massage Chair (MC), bed and other mechanical devices. Electrical massage device treatment has grown in popularity, especially mechanical MCs and beds; their total sales continue to increase worldwide [13].
A comparison of classical Hand Massage (HM) with mechanical massage has shown that mechanical devices have several advantages [14]. A mechanical MC massage has demonstrated effectiveness in controlling pain, improving patient satisfaction and changing their quality of life. According to a study by Kim, et al. [15], mechanical MC therapy was more cost-effective than manual massage. On the other hand, a previous study has shown that MCs had a less positive effect, compared to classical massage procedures [16].
The use of MCs—in Estonia and elsewhere in the world—is gaining momentum. They are frequently purchased for home and office and are used in SPAs, as well as in sports clubs and training centers. However, to date, very little research has been done on the effects of MC massage on muscle tone, biomechanical properties, and recovery from fatigue. The aim of the current research was to elucidate the effects of hand and MC massage on the biomechanical parameters of the muscles of the lower limbs and back, indicators of pressure pain thresholds and subjectively perceived fatigue.
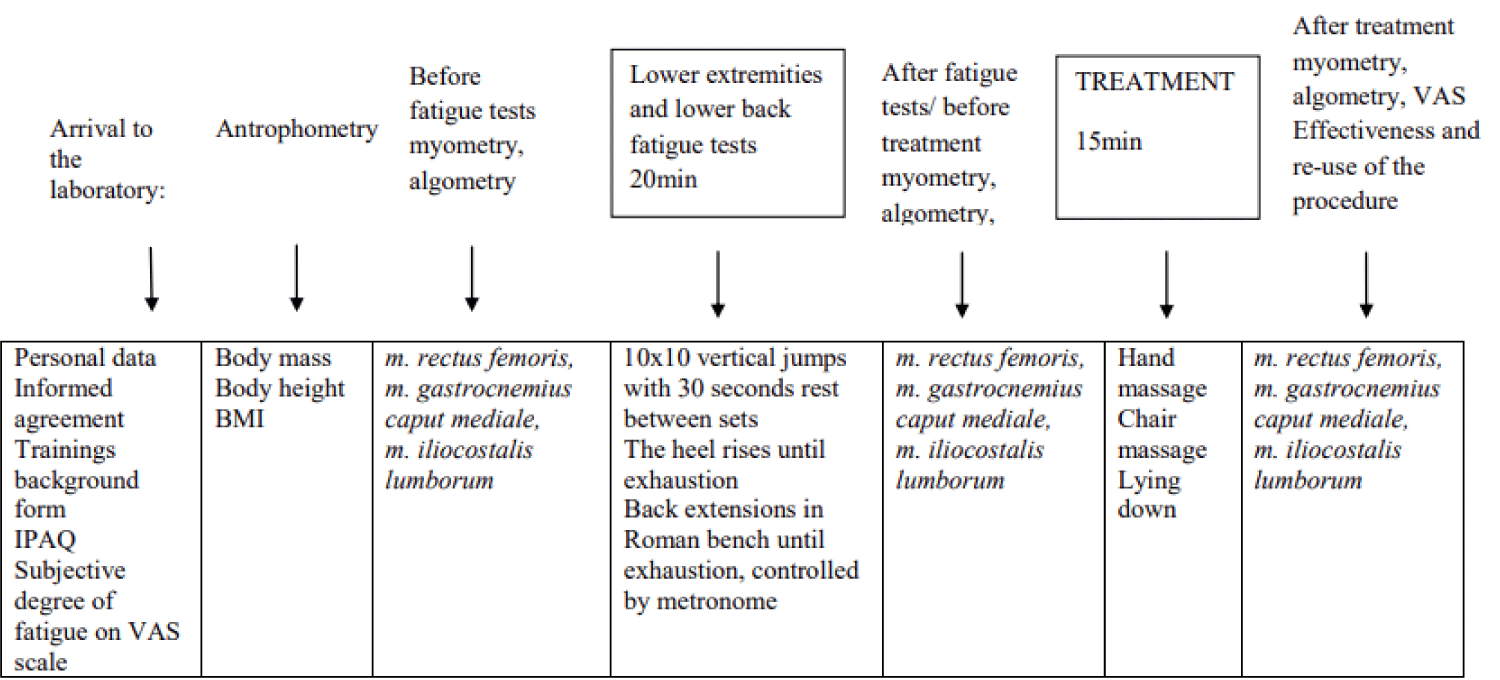
The research subjects were 32 female recreational athletes, aged 18 years - 50 years, who were physically active for at least 150 - 300 min per week. The subjects were asked not to exercise the day before the study. Exclusion criteria were recent injuries, severe pain syndromes, acute inflammations, pregnancy and any oncological, cardiovascular, cardiorespiratory, rheumatic, or other medical conditions that were contraindications to physical capacity testing to avoid deterioration of the health situation. Controlled chronic conditions/diseases were not an absolute contraindication to study participation. The study was approved by the Research Ethics Committee of the University of Tartu, Estonia (protocol no. 347/T-10) and the design of the study is presented in Figure 1.
Figure 1: Study protocol. IPAQ: International Physical Activity Questionnaire; VAS: Visual Analogue Scale; BMI: Body Mass Index.
Questionnaires
A short questionnaire prepared by the study organizers was completed to map the general data (age and health status) and Physical Activity (PA) level. This was the International Physical Activity Questionnaire (IPAQ-SF, validated in Estonian) [17], which examines three specific types of activity undertaken during the previous 7 days. According to the official IPAQ guidelines [18], each item (vigorous intensity, moderate intensity and walking) was summed, in order to estimate the total time spent engaged in PA per week.
A Visual Analogue Scale (VAS) was used to assess the degree of perceived fatigue in the thigh (m. rectus femoris), lower leg (m. gastrocnemius), and lower back (lumbar part of m. iliocostalis lumborum) area. The VAS was a straight horizontal line 100 mm long, marked “no fatigue” at one end and “very severe fatigue” at the other end. The subject marked a place with a vertical line, which characterized the intensity of the fatigue she perceived. The distance between “no fatigue” and the vertical line marked by the subject was measured [19]. To assess the effectiveness of recovery and the subject’s likelihood to reuse the experienced treatment, Likert´s 5 - point scale was used, where 1 indicated complete disagreement with the statement and 5 indicated absolute agreement with the statement.
Anthropometric measurements
During the anthropometric measurements, the subjects were barefoot and wore minimal clothing. Body length was measured while standing using a portable anthropometer (GPM Anthropometrical Instruments, Switzerland; measurement accuracy 5 mm). A digital scale (Soehnle, Germany) with a measurement accuracy of 0.1 kg was used to measure body weight. Body mass index (BMI) was calculated: body weight (kg) / body length (m)2.
Pain pressure threshold
Pain Pressure Threshold (PPT) was measured with an algometer (Wagner Instruments FPK 20, Greenwich, USA). The tip of the algometer was placed perpendicular to the skin surface at the measurement point, and the investigator increased the compression pressure at a rate of 1 kg/s until the pressure sensation was replaced by a slightly unpleasant pain sensation, indicating the PPT (kg/cm2) [20-21]. PPT was measured twice at each point [22], bilaterally: m. gastrocnemius ‘caput mediale’ in the middle of the muscle belly [21], m. rectus femoris in the middle of the muscle belly (the point between the anterior superior spina iliaca anterior superior and the apex of the patella) [23] and at the L3 level, 5 cm laterally of pr. spinosus [24]. The average results of the two measurements were considered [25] and the results of the right and left sides of the body were pooled. All muscle PPT measurements were performed by one assessor.
Muscle biomechanical parameters
The myometric method and a handheld my tonometer (MyotonPro, Myoton Ltd, Estonia) were used to measure muscle: 1) natural oscillation frequency [Hz], which characterizes the muscle tension or tone or muscle biomechanical properties; 2) logarithmic decrement, which characterizes the elasticity of the muscle, i.e., the ability of the muscle to recover to its original shape after contraction; 3) stiffness (N/m), which characterizes the ability of a muscle to resist a force that changed its shape and muscle viscoelastic components; 4) mechanical stress relaxation time (ms); and 5) ratio of deformation and relaxation time, creep (Deborah number) [5].
Lower limb rest natural oscillation frequency [Hz], stiffness (N/m), logarithmic decrement, relaxation time, and creep were measured bilaterally with a myometer (MyotonPro, multiscan - 20 measurement in one-second measurement mode) in the lying position. The following muscles were measured: m. gastrocnemius ‘caput mediale’, the middle point of the m. rectus femoris and lumbar part of m. erector spinae (m. iliocostalis lumborum). All muscle mechanical property measurements were performed by one assessor.
Fatigue protocol
To evoke fatigue in the lower back and lower extremities, the subjects performed 10 series x 10 reps of the maximum vertical jump [26]. The pause between series was 30 s. Going to deep squat, each subject touched the floor with her fingers, followed by a jump with her hands raised.
Subsequently, the subjects completed an exercise to induce fatigue in m. gas-trocnemius, in which the subject stood on one leg on a wooden block and repeatedly lowered and raised the heel from the support surface to exhaustion while keeping the knee joint and torso straight. The exercise was performed with both legs. Each subject was allowed to rest her fingers on the wall at shoulder height to maintain balance. The frequency of the movements was given by metronome (60 movements per minute, i.e., 1 s. concentric and 1 s. eccentric contraction). The exercise was terminated if the subject was 1) exhausted or 2) unable to adhere to the prescribed pace, or 3) unable to maintain balance under given conditions [27].
The third exercise was designed to weaken the lower back extensor muscles (m. erector spinae). Each subject tilted her body in front of and behind the body on a 45 - degree Roman bench, fingers interlaced behind the neck. The exercise was performed to exhaustion at the pace given by the metronome (45 beats per minute, i.e., the duration of the flexion was 1.3333 s and the duration of the extension was 1.3333 s). The exercise was terminated if the subject was unable to 1) perform a body extension with the initial amplitude or 2) adhere to the set pace.
Methodology of different forms of massage
After performing the fatigue test, the subjects were randomly divided into three sub-groups and were allocated either to a) the HM group (n = 11), b) the MC group (n = 11), or c) the calmly lying down group (LD; n = 10). Each group was treated for 15 minutes.
The HM was performed on the lower back, buttocks, and calves. No massage treatments were applied over m. rectus femoris. The HM used classical Swedish massage techniques, i.e., effleurage, petrissage, tapotement and vibration.
The HM lasted 15 minutes. The time was distributed between the body segments as follows: buttocks and lower back (7 min) and lower limbs, i.e., legs and feet (8 min). The MC (Borealis, Ultra Plus, China) provided a 15 - minute automatic program (“Sports Refresh”) that targeted the soles of the feet, the muscles of the lower leg, the buttocks and the lower back [28].
Statistical analysis
The data distribution was checked with Shapiro–Wilk test and the results showed non-normal distribution. Additionally, because the study groups were small in number, data were presented as median values and 25th and 75th percentiles (25; 75). Muscle oscillation frequency, stiffness, decrement and muscle viscoelastic properties (relaxation time and creep) characteristics, as well as pressure pain threshold data, were presented as pooled data of the right and left sides. Changes (∆) in muscle oscillation frequency and in muscle biomechanical parameters (stiffness, decrement) and muscle viscoelastic properties (relaxation time and creep) were calculated as follows: I–II ∆: baseline minus the result after fatigue tests; II–III ∆: the result after the fatigue tests minus the result after the treatment. Kruskal–Wallis and one-way ANOVA were used to compare changes in muscle biomechanical parameters between groups over time. Post-hoc analyses using Bonferroni multiple comparison tests were performed if a significant interaction effect was detected. An alpha level of p < 0.05 was used to determine the statistical significance for all procedures. SPSS (version 26, Chicago, III) was used for analysis.
The anthropometric parameters and PA state of the subjects are presented in Table 1. Measured anthropometric and activity characteristics did not differ between study groups. The age of members of the LD group showed a tendency to differ from the HM group (p = 0.06, after Bonferroni correction).
| Table 1: Descriptive data of the subjects. | |||||||
| Study group | Age (y) | BMI (kg/m2) | IPAQ (MET/min) | Training status (y) | Training per week | Overall fatigue (mm) | |
| HM (n = 11) | 25 (22;26) | 21 (20;22.5) | 2796 (2547;3479) | 7 (4;10) | 3 (2;5) | 1.5(0.5;3.6) | |
| MC (n = 11) | 36 (24;44) | 22 (22; 22.5) | 3260 (2954.5;5094.5) | 9 (2;18) | 4 (3;6) | 3.5(1.0;5.3) | |
| LD (n = 10) | 39 (38;45) | 21 (20;23) | 2565 (2211; 3513) | 3,5 (1;11.23) | 3 (2;4.5) | 2.75(2;6.5) | |
| Values are presented as medians and 25th and 75th percentiles. HM: Hand Massage; MC: Massage Chair; LD: Lying Down; BMI: Body Mass Index; IPAQ: International Physical Activity Questionnaire; MET: Metabolic Equivalent. | |||||||
Degree of perceived fatigue and effectiveness of recovery
For the lower part of the legs (m. gastrocnemius caput mediale: GM), the subjectively assessed perceived fatigue levels after the fatigue tests were close to the maximum result in the HM and LD groups—and did not differ from each other (Table 2). However, in the MC group, the subjectively perceived fatigue rating after the treatment for lower legs (GM) was significantly lower compared to the perceived fatigue rating after the fatigue test in the HM and LD groups (p ˂ 0.001 and p = 0.013, respectively). All study groups rated the experienced recovery procedure as moderately effective. The subjective assessment of the repeated use of the respective treatment in the future was the lowest in the MC group.
| Table 2: Differences in subjectively perceived fatigue between groups and within groups (in VAS scale, mm. | ||||||
| I ICL |
II ICL |
I RF |
II RF |
I GM |
II GM |
|
| HM (n = 11) | 7 (6;9.5)* | 1 (1;3) | 8 (5.5;9.5)* | 2 (1.5;3.5) | 9 (8.5;10)*¥ | 3 (2;5.5) |
| MC (n = 11) | 6 (4.5;7.5)* | 2 (1;3.5) | 4 (2;7)* | 2 (0.5;3) | 6 (4;6.5)‡ | 4 (2.5;6) |
| LD (n = 10) | 5,5 (3;9)* | 1 (0;4) | 8 (7;9)* | 2 (1;6) | 8.5 (7;10)* | 3.5 (1;8) |
| Values are presented as medians and 25th and 75th percentiles. HM: Hand Massage; MC: Massage Chair; LD: Lying Down; I: After Fatigue Tests; II: After Treatment; RF: m. Rectus Femoris; GM: m. Gastrocnemius caput Mediale; ICL: m. Iliocostalis Lumborum; *p ˂ 0.05 I vs. II; ¥p ˂ 0.05 HM vs. MC; ‡p ˂ 0.05 MC vs. LD. | ||||||
The effectiveness of the treatments, in Likert´s scale ratings, did not differ between groups, although the median value of the HM group [5 (3;5)] was higher than the MC [3 (3;4.5)] and LD [3 (3;4)] groups. The ratings for repeated use of treatment in HM were 5 (3;5), 3 (1;5), and 3,5 (2;5) in MC and LD groups, respectively. No differences were observed between groups.
Pressure pain threshold
HM group baseline GM PPT was significantly higher compared to the after-treatment value (p = 0.02). In the MC group, the RF, GM and PPT lowered significantly after treatment (p = 0.02 and p = 0.01, respectively). The MC group’s baseline RF and GM median value of PPT was significantly higher (p = 0.001 and p = 0.02, respectively) than in the LD group. HM group’s baseline PPT, GM and ICL median values were significantly higher than in LD (both p = 0.02; Table 3).| Table 3: Changes in pressure pain threshold with fatigue tests and treatment (kg/cm2). | |||||||||
| I RF | II RF | III RF | I GM | II GM | III GM | I ICL | II ICL | III ICL | |
| HM (n = 11) | 5.6 (4.6;7.1) | 6.1 (4.0;7.4) | 5.5 (4.7;6.2) | 4.9 (3.5;5.8)*† | 4.7 (3.5;5.6) | 4.2 (3.4;6.1) | 7.9 (6;10)† | 7.1 (5.4;10.0) | 7.1 (5.4;10.0) |
| MC (n = 11) | 7 (5.9;9.9)*‡ | 6.6 (5.3;9.1)#‡ | 6.2 (4.2;8.5)‡ | 4.5 (3.5;7.7)‡ | 3.5 (3.1;7.2)# | 3.2 (2.6;5.6) | 6.5 (4.9;9.3) | 7.4 (5.0;9.7) | 6.7 (4.5;10.0) |
| LD (n = 10) | 5.0 (4;5.6) | 5.0 (4;5.7) | 4.7 (3.5;5.6) | 3.6 (2.8;4.1) | 4.0 (3.0; 5.5) | 3.8 (2.9;4.6) | 5.2 (4.1;7.1) | 6.0 (4.3;7.3) | 6.2 (4.3;7.7) |
| Values are presented as medians and 25th and 75th percentiles. HM: Hand Massage; MC: Massage Chair; LD: Lying Down; I: Baseline; II: After Fatigue Tests; III: After Treatment; RF: m. Rectus Femoris; GM: m. Gastrocnemius Caput Mediale; ICL: m. Iliocostalis Lumborum; #p < 0.05 II vs. III; *p < 0.05 I vs. III; †p ˂ 0.05 HM vs. LD; ‡ p ˂ 0.05 MC vs. LD. | |||||||||
After fatigue tests and after treatment, the RF PPT was significantly higher in MC compared to the LD group (p = 0.01 and p = 0.02, respectively).
Muscle biomechanical parameters
The measured muscles’ oscillation frequency, stiffness, decrement, relaxation time and creep (pooled data for the right and left sides) values were characterized by high in-tra-group variability. Table 4 presents the muscles and their biomechanical characteristic median changes (Δ), where statistically significant differences were revealed after post hoc correction between the study groups. Significant changes within the group were also included. There were no between-group differences in muscle oscillation frequency and logarithmic decrement changes for any measured muscle (data not presented in the table).
| Table 4: Comparison of median differences between and within study groups. | ||||||
| I-II ΔGM S |
II-III ΔGM S |
I-II ΔGM trelax |
II-III ΔGM trelax |
I-II ΔGM C |
II-III ΔGM C |
|
| HM (n = 11) | 1 (-13;14)¤ | 19.5 (5;32.3)¥ | 0.3 (-0.6;1.25)¤ | -0.7 (-1.9;0.1) | 0.05 (-0.03;0.07) | -0.02 (-0.08;0.06) |
| MC(n = 11) | 3.5 (-3.3;22.3) | 0 (-15;8) | 0.1 (-1.1;0.8) | 0 (-1.1;1.6) | 0.02 (-0.04;0.05) | 0.01 (-0.07;0.09) |
| LD (n = 10) | 7 (-3.8;21.5) | 8.5 (1.5;15.5) | -0.25 (-1.55;0.8) | -1 (-1.4; -0.1) | -0.015 (-0.08;0.06) | -0.05 (-0.09;0.01) |
| I-II ΔICL S |
II-III ΔICL S |
I-II ΔICL trelax |
II-III ΔICL trelax |
I-II ΔICL C |
II-III ΔICL C |
|
| HM (n = 11) | -11,5 (-54.5;15.5) | 4 (-21.5;65.3) | 0.6(-0.4;2.2) | -0.2(-1.6;0.6) | 0.1(0.01;0.02)¤ | -0.01 (-0.1;0.04)¥ |
| CM (n = 11) | 7 (-1.5;16.6) | 6 (-9;14.3) | 1,2 (0.03;2.3)¤ | -1.6(-2.7; -0.7) | 0.1 (0.02;0.2)¤ | -0.1 (-0.2; -0.1) |
| LD(n = 10) | 10,5 (-19.5;24.5) | 1 (-8,8;27.5) | 1.9(0.1;4.2)¤ | -1.3(-3.3;0.6) | 0.1 (0.01;0.3)¤ | -0.1 (-0.2;0) |
| I-II ΔRF S |
II-III ΔRF S |
I-II ΔRF trelax |
II-III ΔRF ttrelax |
I-II ΔRF C |
II-III ΔRF C |
|
| HM(n = 11) | -4.5 (-12.5;0.8)¤ⴕ | 6.5 (1.75;12.8) | 0.8(0.3;0.9)¤ⴕ | -0.4 (-1.2; -1.0) | 0,04 (0,02;0.1)¤ | -0,02 (-0.01;0.0) |
| CM(n=11) | 0.5 (7;4) | 4 (-1.25;8) | 0.6 (0.03;0.9)¤ | -0.4 (-0.6;0.03) | 0,04 (0;0.01)¤ | -0,02 (-0.1;0.02) |
| LD(n = 10) | 4 (-8.75;12.3) | 6 (0;11) | 0.1 (-0.7;0.9) | -0.56 (-1.4;0.05) | 0,01 (-0,04;0.01) | -0.02 (-0.1;0.01) |
| Values are presented as medians and 25th and 75th percentiles. HM: Hand Massage; MC: Massage Chair; LD: Lying Down; RF: m. Rectus Femoris; GM: m. Gastrocnemius Caput Mediale; ICL: m. Iliocostalis Lumborum; S: Stiffness; trelax: Relaxation Time; C: Creep; I–II: Baseline – After Fatigue Tests; II–III: After Fatigue Tests - After Treatment. ¤p < 0.05 I–II vs. II–III; ⴕp < 0.05 HM vs. LD; ⱡp < 0.05 MC vs. LD; ¥p < 0.05 HM vs. MC. |
||||||
In the case of GM muscle, significant changes between groups were expressed in three biomechanical parameters (stiffness, relaxation time, creep). The change in GM stiffness (II–III ΔGM; Table 4) caused by the treatment was more extensive in the case of HM, which was significantly different (p = 0.002) from the change manifested in the MC group (Table 4). Within the HM group, the change in GM stiffness with treatment was significant compared to the change with the fatigue tests (lowered with treatment), which was not observed for GM stiffness in the other study groups. In the MC group, the change in GM stiffness with the treatment stayed the same. This was not significantly different from the LD group.
The GM relaxation time change with fatigue tests did not differ between study groups. Within the HM group, GM relaxation time lengthened significantly with treatment (p = 0.01). The changes in ICL creep with fatigue tests were similar (significantly reduced; p = 0.01; p = 0.02; p = 0.01 HM, MC, and LD respectively) for all study groups and did not differ between groups (Table 4). With the treatment, ICL creep lengthened in all groups, differing between HM and MC groups (p = 0.03). Changes in RF stiffness with fatigue tests were significantly higher in HM compared with the LD group (p = 0.03). In the HM group, the RF stiffness change with fatigue tests decreased significantly, in comparison with RF stiffness change with treatment (p = 0.02). The RF relaxation time change was significantly shorter with fatigue tests in the HM group than in the LD group (p = 0.03). Within the HM and CM groups, the changes in RF relaxation time and creep with treatment were statistically significant, compared to the changes with fatigue tests (p = 0.01; p = 0.03 and p = 0.03; p = 0.04, respectively).
The main finding of this study was that the values of muscle biomechanical parameters at rest exhibited significant interindividual variability for all muscles. We did not find typical patterns of muscle behavior in any biomechanical parameter with fatigue tests. Judging by the behavior of the muscle biomechanical parameters, we concluded that the fatigue test did not induce sufficient fatigue in the observed muscle groups, since we did not find an increase in muscle stiffness, tone, and decrement, as has been shown previously [29-31], or the accompanying shortening in muscle relaxation time and creep [31]. Although, Klich, et al. [31] obtained a significant increase in biomechanical parameters of lower limb muscles with a decrease in muscle viscoelastic properties (trelax and creep) after repeated 200m sprints on a bicycle, which is biomechanically logical. In our study, such logic was clearly seen in the case of the ILC and RF muscle groups only in the HM group, where the change in stiffness after the fatigue test had an upward trend and was accompanied by a shortening of the relaxation time and a decrease in creep. However, with the treatment, i.e., HM, mostly the opposite changes took place. In other study groups, this expected behavior of the measured parameters was less pronounced. This result may have been related to the individuality of the subjects (including differences in age, physical training, and training characteristics). It was found that age affected the biomechanical properties of different muscles [32-34]. We did not find differences between our study groups in any baseline measurement of biomechanical properties, although the LD group tended (p = 0.06) to be a little bit older than the other two study groups. Although the loss of muscle mass has typically already begun after the age of 30, it becomes significant only after the age of 60 [35]. Similarly, changes in the biomechanical properties of muscle become more noticeable after the age of 60 [32]. It is known that age-related changes in muscle mass can be mitigated by regular physical activity and optimal nutrition [36], and thus it could be assumed that it would also be possible to reduce the speed of changes in the biomechanical properties of the muscle.
Looking at intra - group changes in the behavior of biomechanical parameters, the changes induced by the fatigue test were not in the same direction within the study groups. The subjects were divided into groups at random. Thus, it is possible that one group was formed by subjects whose muscle biomechanical parameters in one or another muscle were not similar at the baseline (initial) level of the entire group, and that reactions to fatigue testing were likewise dissimilar or vice versa.
Muscle fatigue could best be reflected by an increase in muscle stiffness. A significant increase in muscle stiffness was documented by Banerjee, et al. [29] immediately after forearm curls with a 3 kg dumbbell till exhaustion and/or failure of the task. Kong, et al. [30], however, found that after 40 min of downhill running, lower limb muscle stiffness increased only 24, 48, 72, and 96 h after finishing the downhill run, but not immediately after the run or post-massage. In our study, the changes induced by the fatigue test were not in the same direction in any of the study groups. In the HM group, the most logical changes in biomechanical parameters appeared with the fatigue tests and treatment. For example, in the case of RF stiffness, the stiffness in the HM group increased with the fatigue test (delta negative), which was also accompanied by a shortening of the relaxation time and a drop in RF creep. Results were nearly the same with GM and ICL stiffness in the HM group. At the same time, in CM and LD groups, RF and GM stiffness decreased, and the relaxation time lengthened with the fatigue test, contrary to the results of previous studies [29,31]. Unfortunately, we did not objectively control the performance of the fatigue tests. However, Klich, et al. [31] obtained a 25.3% decrease in jumping performance, compared to baseline, with a similar fatigue test (14 x 10 jumps up with 1 min rest), although they did not measure muscle biomechanical parameters. It could be that we did not measure at the right time, i.e., when fatigue was most severe. Kong, et al. [30] recorded the greatest increase in stiffness only 24 hours after 40 minutes of downhill running, although Banerjee, et al. [29] and Klich, et al. [31] recorded increases in stiffness immediately after activity. Unfortunately, we did not measure blood biochemical biomarkers [30,37]. Additionally, we did not measure muscle strength or decreases in muscle strength that could reflect muscle fatigue [38].
In terms of muscle tone (oscillation frequency) and elasticity (logarithmic decrement) of the measured muscles (RF, GM, ICL), it did not matter which recovery tool was used. However, HM decreased RF and GM stiffness, lengthened relaxation time and increased creep in the HM group, indicating that HM could be effective in reducing GM and RF stiffness, as compared to MCs. ICL creep increased with treatment for all study groups, which could express a decrease in stiffness and a lengthening in relaxation time. In the MC group, the ICL creep increase with treatment was the most extensive and significantly differed from the same indicator in the HM group. It could suggest that MC led to the most extensive increase in creep, which could mean that the stiffness of the muscle also decreased and that relaxation time was lengthened.
The results could indicate the effectiveness of HM in reducing muscle stiffness, as a change in RF, GM and ICL stiffness (delta negative) showed that the direction of stiffness increased with fatigue tests and decreased with treatment (ICL, not significantly) in the HM group. Although RF did not receive any treatment. Rather, when designing the study, RF was excluded in the case of HM, because in the case of CM, this muscle was not directly affected. We assumed that the indirect effects of massage [30] through the massage of neighboring muscles played a role in such a decrease in RF stiffness in the HM group. It could also be assumed that such an effect was not manifested in the case of MC message because, in the case of MC massage, the neighboring muscles were continuously affected for 15 min—while in the case of HM, each muscle group was only affected around 3.75 min. An MC massage could be too intense and prolonged to induce muscle relaxation. In addition, in the MC group, RF did not show muscle fatigue under the influence of fatigue tests. This could be due to the inadequacy of our designed fatigue tests, or perhaps another head of m. quadriceps femoris could have been more affected by the exercise.
The subjectively given assessments showed that the fatigue protocol applied in this study induced muscle fatigue with the corresponding tests and recovered with the corresponding treatments, but these results did not match with the objectively measured muscle biomechanical parameter results. Hand massage was rated, although not significantly, as the most effective and most likely to experience the procedure again. This could be because HM had a positive psychological effect to some extent [39], although no clear evidence for a beneficial psychological effect has been observed [37]. Kim, et al. [15] also pointed to the importance of human contact in the therapy outcome.
Expectedly, PPT could decrease with fatigue tests and increase again with treatment when the muscle has recovered. Cyganska, et al. [40] found a significant increase in the PPT after each HM procedure and meeting by meeting over four months. However, in the control group, the PPT decreased, instead. In our study, the PPT in the HM group for GM decreased significantly with treatment and there was no significant increase in the PPT in any of the study groups. Obviously, this could be attributed to the differences between our study design and subjects and those of Cyganska, et al. [40]. We did not directly search for or affect trigger points with treatment and our study was short-term compared to Cyganska, et al. [40].
These results suggested that, in the future, study design with respect to subjects could be more explanatory. Additionally, representatives of the same sport and age group could be used in further analogous studies. Lastly, individuals with similar muscle biomechanical parameters should be grouped into one study group. Current study results suggest the need for larger randomized controlled trials of MC versus other massage techniques. The authors of this article are already planning a larger-scale study with the above-mentioned adaptations in the fall of 2023.
One limitation of this study was the fact that the structure of the muscle-fatiguing test did not fulfill its purpose, although the muscle work was equal in concentric and eccentric modes. Certainly, more attention should be paid to the sample size and the homogeneity of the study group (including representatives of the same sport). Likewise, attention must be paid to the muscles under study, e.g., which part of the muscle carries the main load, so that fatigue could be more pronounced after the fatigue tests (m. vastus lateralis besides m. rectus femoris). More complete assessment methods (tensiometer, EMG, biochemical markers) would be useful, in addition to myometry, to better explain the processes inside given muscles. At the same time, this study was a step forward in research on HM versus MC using different research methods (both subjective and objective). The impacts of fatigue testing can be very individual, and treatments to speed up recovery are not always needed. One strength of our study lay in the fact that we used a control group, i.e., one in which the subjects were asked to simply lie down and did not receive any treatment.
Hand massage may have benefits for recovery from physical exertion. However, due to the individuality of people (including within the muscles), detailed methodological studies are needed to evaluate the effects of MC massage vs. HM. It must also be considered that different methods for recovery from physical exertion may be suitable to different people due to individuality—any generalization may be incorrect.
The authors wish to express their sincere appreciation to the study participants and to Borealis Estonia LLC for the supply of massage chairs for the study.
- Minett GM, Duffield R. Is recovery driven by central or peripheral factors? A role for the brain in recovery following intermittent-sprint exercise. Front Physiol. 2014 Feb 3;5:24. doi: 10.3389/fphys.2014.00024. PMID: 24550837; PMCID: PMC3909945.
- Hausswirth C, Le Meur Y. Physiological and nutritional aspects of post-exercise recovery: specific recommendations for female athletes. Sports Med. 2011 Oct 1;41(10):861-82. doi: 10.2165/11593180-000000000-00000. PMID: 21923203.
- Butler RJ, Crowell HP 3rd, Davis IM. Lower extremity stiffness: implications for performance and injury. Clin Biomech (Bristol, Avon). 2003 Jul;18(6):511-7. doi: 10.1016/s0268-0033(03)00071-8. PMID: 12828900.
- Wang JS. Therapeutic effects of massage and electrotherapy on muscle tone, stiffness and muscle contraction following gastrocnemius muscle fatigue. J Phys Ther Sci. 2017 Jan;29(1):144-147. doi: 10.1589/jpts.29.144. Epub 2017 Jan 30. PMID: 28210061; PMCID: PMC5300827.
- Vain A, Toomla T, Kahn KH. Relationship of skeletal muscle biomechanical parameters determined by the myometry method with arterial hypertension. Estonian Doctor. 2006; 85(1):14–19. doi: https://doi.org/10.15157/ea.v0i0.10065.
- Torres R, Ribeiro F, Alberto Duarte J, Cabri JM. Evidence of the physiotherapeutic interventions used currently after exercise-induced muscle damage: systematic review and meta-analysis. Phys Ther Sport. 2012 May;13(2):101-14. doi: 10.1016/j.ptsp.2011.07.005. Epub 2011 Sep 14. PMID: 22498151.
- Dupuy O, Douzi W, Theurot D, Bosquet L, Dugué B. An Evidence-Based Approach for Choosing Post-exercise Recovery Techniques to Reduce Markers of Muscle Damage, Soreness, Fatigue, and Inflammation: A Systematic Review With Meta-Analysis. Front Physiol. 2018 Apr 26;9:403. doi: 10.3389/fphys.2018.00403. PMID: 29755363; PMCID: PMC5932411.
- Leeder J, Gissane C, van Someren K, Gregson W, Howatson G. Cold water immersion and recovery from strenuous exercise: a meta-analysis. Br J Sports Med. 2012 Mar;46(4):233-40. doi: 10.1136/bjsports-2011-090061. Epub 2011 Sep 22. PMID: 21947816.
- Bieuzen F, Brisswalter J, Easthope C, Vercruyssen F, Bernard T, Hausswirth C. Effect of wearing compression stockings on recovery after mild exercise-induced muscle damage. Int J Sports Physiol Perform. 2014 Mar;9(2):256-64. doi: 10.1123/ijspp.2013-0126. Epub 2013 May 22. PMID: 23751727.
- Herbert RD, Gabriel M. Effects of stretching before and after exercising on muscle soreness and risk of injury: systematic review. BMJ. 2002 Aug 31;325(7362):468. doi: 10.1136/bmj.325.7362.468. PMID: 12202327; PMCID: PMC119442.
- Field T. Massage therapy research review. Complement Ther Clin Pract. 2014 Nov;20(4):224-9. doi: 10.1016/j.ctcp.2014.07.002. Epub 2014 Aug 1. PMID: 25172313; PMCID: PMC5467308.
- Visconti L, Capra G, Carta G, Forni C, Janin D. Effect of massage on DOMS in ultramarathon runners: A pilot study. J Bodyw Mov Ther. 2015 Jul;19(3):458-63. doi: 10.1016/j.jbmt.2014.11.008. Epub 2014 Nov 24. PMID: 26118518.
- Zhong H, Eungpinichpong W, Wang X, Chatchawan U, Wanpen S, Buranruk O. Effects of mechanical-bed massage on exercise-induced back fatigue in athletes. J Phys Ther Sci. 2018 Mar;30(3):365-372. doi: 10.1589/jpts.30.365. Epub 2018 Mar 2. PMID: 29581653; PMCID: PMC5857440.
- Muller J, Handlin L, Harlén M, Lindmark U, Ekström A. Mechanical massage and mental training programmes affect employees' anxiety, stress susceptibility and detachment-a randomised explorative pilot study. BMC Complement Altern Med. 2015 Sep 2;15:302. doi: 10.1186/s12906-015-0753-x. PMID: 26329694; PMCID: PMC4556221.
- Kim SK, Min A, Jeon C, Kim T, Cho S, Lee SC, Lee CK. Clinical outcomes and cost-effectiveness of massage chair therapy versus basic physiotherapy in lower back pain patients: A randomized controlled trial. Medicine (Baltimore). 2020 Mar;99(12):e19514. doi: 10.1097/MD.0000000000019514. PMID: 32195952; PMCID: PMC7220115.
- Siško PK, Videmšek M, Karpljuk D. The effect of a corporate chair massage program on musculoskeletal discomfort and joint range of motion in office workers. J Altern Complement Med. 2011 Jul;17(7):617-22. doi: 10.1089/acm.2010.0400. Epub 2011 Jun 20. PMID: 21688984.
- International Physical Activity Questionnaire Web site. 29 January 2022. http://www.ipaq.ki.se
- Scoring the International Physical Activity Questionnaire. 29 January 2022. https://bit.ly/2tRmOaR
- Leung AW, Chan CC, Lee AH, Lam KW. Visual analogue scale correlates of musculoskeletal fatigue. Percept Mot Skills. 2004 Aug;99(1):235-46. doi: 10.2466/pms.99.1.235-246. PMID: 15446651.
- Arroyo-Morales M, Olea N, Martínez MM, Hidalgo-Lozano A, Ruiz-Rodríguez C, Díaz-Rodríguez L. Psychophysiological effects of massage-myofascial release after exercise: a randomized sham-control study. J Altern Complement Med. 2008 Dec;14(10):1223-9. doi: 10.1089/acm.2008.0253. PMID: 19123877.
- Aspinall SL, Leboeuf-Yde C, Etherington SJ, Walker BF. Changes in pressure pain threshold and temporal summation in rapid responders and non-rapid responders after lumbar spinal manipulation and sham: A secondary analysis in adults with low back pain. Musculoskelet Sci Pract. 2020 Jun;47:102137. doi: 10.1016/j.msksp.2020.102137. Epub 2020 Feb 26. PMID: 32148330.
- Gajsar H, Titze C, Hasenbring MI, Vaegter HB. Isometric Back Exercise Has Different Effect on Pressure Pain Thresholds in Healthy Men and Women. Pain Med. 2017 May 1;18(5):917-923. doi: 10.1093/pm/pnw176. PMID: 27473635.
- Anaya-Terroba L, Arroyo-Morales M, Fernández-de-Las-Peñas C, Díaz-Rodríguez L, Cleland JA. Effects of ice massage on pressure pain thresholds and electromyography activity postexercise: a randomized controlled crossover study. J Manipulative Physiol Ther. 2010 Mar-Apr;33(3):212-9. doi: 10.1016/j.jmpt.2010.01.015. PMID: 20350675.
- Farasyn A, Meeusen R. Pressure pain thresholds in healthy subjects: influence of physical activity, history of lower back pain factors and the use of endermology as a placebo-like treatment. J Bodyw Mov Ther. 2003 Jan;7(1):53–61. doi: 10.1016/S1360-8592(02)00050-5.
- Keating L, Lubke C, Powell V, Young T, Souvlis T, Jull G. Mid-thoracic tenderness: a comparison of pressure pain threshold between spinal regions, in asymptomatic subjects. Man Ther. 2001 Feb;6(1):34-9. doi: 10.1054/math.2000.0377. PMID: 11243907.
- Knihs DA, Zimmermann HB, Pupo JD. Acute and Delayed Effects of Fatigue on Ground Reaction Force, Lower Limb Stiffness and Coordination Asymmetries During a Landing Task. J Hum Kinet. 2021 Jan 29;76:191-199. doi: 10.2478/hukin-2021-0054. PMID: 33603934; PMCID: PMC7877279.
- Hébert-Losier K, Wessman C, Alricsson M, Svantesson U. Updated reliability and normative values for the standing heel-rise test in healthy adults. Physiotherapy. 2017 Dec;103(4):446-452. doi: 10.1016/j.physio.2017.03.002. Epub 2017 Mar 21. PMID: 28886865.
- Roche N, Crichton ML, Goeminne PC, Cao B, Humbert M, Shteinberg M, Antoniou KM, Ulrik CS, Parks H, Wang C, Vandendriessche T, Qu J, Stolz D, Brightling C, Welte T, Aliberti S, Simonds AK, Tonia T, Chalmers JD. Update June 2022: management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. Eur Respir J. 2022 Aug 10;60(2):2200803. doi: 10.1183/13993003.00803-2022. PMID: 35710264; PMCID: PMC9363848.
- Banerjee SS, Krishnamani DB, Karthick PA, Arunachalakasi A, Swaminathan R. Influence of viscoelasticity on dynamic fatiguing behavior of muscle using myotonometry and surface electromyography measurements. IEEE Transactions on Instrumentation and Measurement 2022 Sep;71. doi: 10.1109/TIM.2022.3205645.
- Kong PW, Chua YH, Kawabata M, Burns SF, Cai C. Effect of Post-Exercise Massage on Passive Muscle Stiffness Measured Using Myotonometry - A Double-Blind Study. J Sports Sci Med. 2018 Nov 20;17(4):599-606. PMID: 30479528; PMCID: PMC6243630.
- Klich S, Krymski I, Kawczynski A. Viscoelastic properties of lower extremity muscles after track cycling sprint events: a case report. CEJSSM. 2020 29:5–10. doi: 10.18276/cej.2020.1-01.
- Agyapong-Badu S, Warner M, Samuel D, Stokes M. Measurement of ageing effects on muscle tone and mechanical properties of rectus femoris and biceps brachii in healthy males and females using a novel hand-held myometric device. Arch Gerontol Geriatr. 2016 Jan-Feb;62:59-67. doi: 10.1016/j.archger.2015.09.011. Epub 2015 Oct 23. PMID: 26476868.
- Kocur P, Tomczak M, Wiernicka M, Goliwąs M, Lewandowski J, Łochyński D. Relationship between age, BMI, head posture and superficial neck muscle stiffness and elasticity in adult women. Sci Rep. 2019 Jun 11;9(1):8515. doi: 10.1038/s41598-019-44837-5. PMID: 31186509; PMCID: PMC6559965.
- Wu Z, Wang Y, Ye Z, Guan Y, Ye X, Chen Z, Li C, Chen G, Zhu Y, Du J, Chen G, Liu W, Xu X. Effects of Age and Sex on Properties of Lumbar Erector Spinae in Healthy People: Preliminary Results From a Pilot Study. Front Physiol. 2021 Sep 20;12:718068. doi: 10.3389/fphys.2021.718068. PMID: 34616306; PMCID: PMC8488426.
- Holloszy JO. The biology of aging. Mayo Clin Proc. 2000 Jan;75 Suppl:S3-8; discussion S8-9. PMID: 10959208.
- Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004 Jul;7(4):405-10. doi: 10.1097/01.mco.0000134362.76653.b2. PMID: 15192443; PMCID: PMC2804956.
- Robertson A, Watt JM, Galloway SD. Effects of leg massage on recovery from high intensity cycling exercise. Br J Sports Med. 2004 Apr;38(2):173-6. doi: 10.1136/bjsm.2002.003186. PMID: 15039254; PMCID: PMC1724761.
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001 Oct;81(4):1725-89. doi: 10.1152/physrev.2001.81.4.1725. PMID: 11581501.
- Hemmings B, Smith M, Graydon J, Dyson R. Effects of massage on physiological restoration, perceived recovery, and repeated sports performance. Br J Sports Med. 2000 Apr;34(2):109-14; discussion 115. doi: 10.1136/bjsm.34.2.109. PMID: 10786866; PMCID: PMC1724183.
- Cygańska A, Truszczyńska-Baszak A, Tomaszewski P. Impact of Exercises and Chair Massage on Musculoskeletal Pain of Young Musicians. Int J Environ Res Public Health. 2020 Jul 16;17(14):5128. doi: 10.3390/ijerph17145128. PMID: 32708600; PMCID: PMC7400366.