More Information
Submitted: March 08, 2024 | Approved: March 22, 2022 | Published: March 25, 2024
How to cite this article: Matheron E. Management of Non-contact Injuries, Nonspecific Chronic Pain, and Prevention via Sensory Conflicts Detection: Vertical Heterophoria as a Landmark Indicator. J Nov Physiother Rehabil. 2024; 8: 005-013.
DOI: 10.29328/journal.jnpr.1001057
Copyright License: © 2024 Matheron E. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abbreviations: VO: Vertical Heterophoria; VH: Vertical Heterophoria; TMJ: TemporoMandibular Joint; CI: Confidence Intervals.
Management of Non-contact Injuries, Nonspecific Chronic Pain, and Prevention via Sensory Conflicts Detection: Vertical Heterophoria as a Landmark Indicator
Eric Matheron*
Physical Therapy office, Dijon, France
*Address for Correspondence: Eric Matheron, Physical Therapy Office, 3 Place du Rosoir, 21000 Dijon, France, Email address: [email protected]
Sensory and sensorimotor conflicts can lead to sensory and motor efficiency disturbances, such as pain and less efficient motor control. Vertical heterophoria (VH) and vertical orthophoria (VO) are respectively the latent vertical misalignment of the eyes when the retinal images are dissociated, or not. Mild VH (< 0.57°) could indicate the presence of a conflict resulting from eye refraction problems and/or a disruption of the somaesthetic cues. Canceling the conflict(s) can immediately restore VO, making it possible to observe an improvement in the mobility of spinal and peripheral joints, the performance in the motor and balance tests after initial alternation, and a decrease in pain. The Maddox Rod Test was used to detect mild VH but doesn’t determine the sensory conflict origin. The aim of this retrospective study is to show its use as a landmark in which sensory afferent conflict could induce symptoms (i.e. pain; decreased range of motion; nonoptimal postural and motor control) and how to manage it, analyzing data from 525 subjects. The clinical process is intended to inhibit or neutralize afferent signals involved in the sensorimotor loops required by the central nervous system in motor control in order to spot the locus of conflict (stomatognathic system, pelvis, plantar afferences, piercings (body art) or/and eye refraction problems). This investigation protocol based on VH detection provides trackers for the therapeutic intervention in the management of nonspecific chronic pain, non-contact injuries, and prevention, and a key role for practitioners in the multidisciplinary management required for patients/athletes, in the world of work/health.
Nonspecific pain and functional limitations following non-contact injuries are frequent complaints in clinical practice. Although they could have numerous origins, we know since the seminal paper of Waddell [1] that nonspecific pain is not often linked with mechanical problems (i.e. imagery linked) and should be managed in a biopsychosocial approach. Introducing the distinction between pain and disability, this author and many clinicians and researchers since him argue for the need to address the psychological and social aspects of the patient’s condition in clinical reasoning. As an aftermath of this school of thought, current researches often omit the biological component [2].
Although mechanical problems are not a pertinent way to classify pain etiologies, other structural deficiencies could be seen in patients suffering from light but constant disruptions in muscular tone. Indeed, it has been reported that nonspecific chronic pain and non-contact injuries could be linked to sensorimotor conflicts/sensory disturbances: Harris suggested, as of 1999 [3], that incongruence between motor intention, proprioception, and vision could lead to the sensation of pain, as incongruence between vision and vestibular sensations lead to the sensation of nausea. The hypothesis has been confirmed by McCabe, et al. [4], inducing pain and other sensations by modulating sensory-motor incongruence in healthy volunteers. They showed that motor-sensory conflict can exacerbate pain in patients with fibromyalgia to a greater extent than in healthy volunteers [5]. In the same way, nonspecific chronic pain could be linked to binocular vision impairments in the vertical direction [6-8]. Vision and gaze signals are known to be involved with vestibular and somaesthetic inputs, in the sensorimotor/perception-action loops for posture, balance, and movement control [9,10]. Vertical heterophoria (VH) is the relative vertical deviation of the visual axes when the binocular vision is interrupted, reduced via binocular vision mechanisms, and vertical orthophoria (VO) without misalignment [11].
In the absence of neurological or vestibular pathologies, it was reported that VH, even when small in size (< 1 diopter, i.e. 0.57°), could indicate the presence of a conflict resulting from eye refraction problems or a disruption of the somaesthetic cues (e.g from the stomatognathic system or pelvis cues) required in the sensorimotor loops involved in motor control and the capacity of the central nervous system to integrate these cues optimally [8].
Interestingly, clinical studies showed that canceling the conflict restored VO immediately most of the time, diminished pain, and made it possible to observe an improvement in the mobility of spinal and peripheral joints, and of the performance in the balance tests after initial alternation [7,12,13]. In this way using the Maddox Rod Test, an easy and cheap test, for VH detection could be a potential landmark in the management of nonspecific chronic pain, non-contact injuries and prevention.
Aim and design
This study is based on an observational cross-sectional retrospective study where the data of 525 subjects - for whom the Maddox Rod Test was practicable and revealed a mild VH - was collected, held anonymously, and analyzed. The aim is to describe the investigation protocol used, the different implicated regions found in the detected VH to obtain the VO, and how to proceed. Indeed, mild VH has been reportedly linked to eye refraction problems such as minor astigmatism or hypermetropia [11,14] and/or sensory afference disturbance from: the stomatognathic system and the pelvis [12,13,15], the sole of the foot [16], or the skin pierced with jewelry (body art), other than in the lobe at least on face and ears [7]. The protocol was carried out in agreement with the legal and international requirements of the Declaration of Helsinki.
Subjects
From the 600 subjects investigated (376 females and 224 males in the age range of 6–93 years, mean age 39.2 ± 17.4 years) 75 subjects were VO or did not test practicable (visual problem, amblyopia, palsy, understanding), this retrospective analysis concerned 525 subjects examined consecutively with VH detected and measured (< 1 diopter) with the Maddox Rod Test (see below). These subjects were investigated for various reasons in physical therapy offices, medical offices, or sports health structures. Firstly, for nonspecific chronic pain with additional comorbidity (e.g. back pain, peripheral arthralgia, tendinopathy, headache, temporomandibular joint disorders, dizziness, dyspareunia, pudendal neuralgia), i.e. lasting longer than 3 months according to the International Association for the Study of Pain criteria where medical consultation and complementary examination did not report anatomical findings, neuropathy or rheumatism [17], and secondly, for non-contact injuries (e.g. tendinopathy, muscle pain); thirdly, for prevention mainly among top class athletes (essentially athletics, basketball, football/soccer, rugby, ice hockey, winter sports, swimming, and tennis) from different countries. In this latter case, the objective was to detect whether sensory conflicts could be present, which could lead to non-optimal motor efficiency and energetic cost at least to maintain quite upright reference posture or rotation stresses at the spine or limbs level [18,19].
Maddox rod test
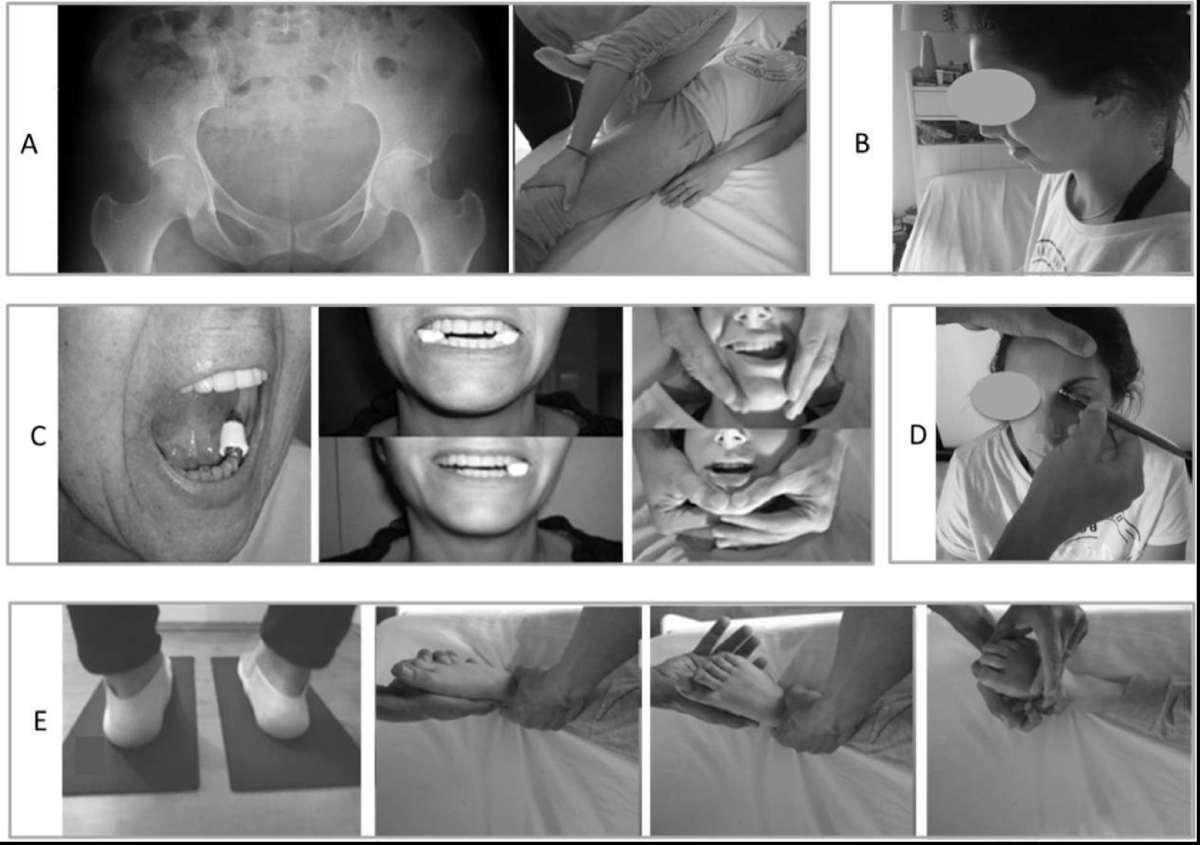
The Maddox Rod Test is appropriate for detecting in free space and measuring clinically the mild vertical deviation or heterophoria of less than one diopter similarly for the objective recording of eye alignment by the magnetic search coil, while the cover test (objective test) may fail [20-22]. It was run as follows in a dimly lit room. The subject stood erect barefoot, more than 2 m in front of a point of light at the level of his/her eyes, in an anatomically upright referenced posture with the arms at his/her side, head straight, and the Frankfort (orbitomeatal) plane horizontal [23]. A skew deviation of the head or a slight modification of the gaze direction can induce VH [21,24]. The red Maddox rod, with its stripes running vertically, was placed in front of one eye transforming this point into a red line until the subject perceived it as horizontal; the other eye saw the point. The presence of VH was concluded when the line was not perfectly seen in the middle of the light point, i.e. upwards or downwards, above or below, respectively called hypophoria and hyperphoria (Figure 1).
Figure 1: The Maddox Rod Test during upright stance.
Next, after normal binocular vision for a short time, the Maddox rod was placed in front of the other eye; von Noorden reported that for various reasons, responses can be different [25]. For instance, for the same subject, if one can have right hyperphoria and left hypophoria, surprisingly right hyperphoria and left orthophoria is possible as is right hyperphoria and left hyperphoria [7,18]. The Maddox Rod Test explores the central retina of the eye focusing on the point of light, and the peripheral retina under the rod [25]. This could explain the different responses. It has been proposed that this easy test can investigate the coherence between the ventral stream and the dorsal stream [26], visual pathways for perception and action, respectively from the central retinal and the peripheral retinal [27,28], perception and action whose prerequisites are the control of body segment orientation and body stabilization, functions of postural control [26,29].
The Maddox Rod Test was carried out with natural vision or with contact lenses if it was the case. If subjects wore glasses, the test was run with them worn only at the beginning. Then, the glasses were removed for a new test (if possible) and the investigation protocol (see below). Indeed, glasses can induce a prism effect.
Investigation protocol
The bilateral restitution of the VO, controlled by the Maddox Rod Test, makes it possible to check that the “neutral/natural” upright reference posture is reached when it was in question, as initially revealed by the presence of VH [7,18]. This restitution appears to be a good criterion for the efficiency of diagnostic and therapeutic maneuvers; the criterion had to be combined with other static and dynamic postural clinical tests such as head or spine active rotation, hip/lower limb passive flexion or rotation, shoulder/upper limb active flexion or abduction, temporomandibular joint (TMJ) opening movement [12,13,15]. However, this is beyond the topic at hand.
The modalities of the following protocol to obtain bilateral VO were sometimes complementary but non-interchangeable [16] provided that they did not seek to stimulate afferences with adapted prisms to cancel VH or other, as experimentally carried out on postural control in subjects with mild VH [7,8,18], and in a clinical way in dyslexics [30], or in patients with persistent post-concussive symptoms [31], for whom VH is present.
The investigation was done until bilateral VO was obtained (i.e. VO when the Maddox rod was run on the right eye and on the left eye), apart from an interaction of vision (see below). Each step of the following protocol was checked with the Maddox Rod Test. When the bilateral restitution of the VO was achieved or a visual input disorder was “neutralized”, static and dynamic postural clinical tests were checked. This is not developed here, just broached. Please note that each VH configuration change (e.g. right hyperphoria and left hypophoria becomes right orthophoria and left hyperphoria) is considered a present conflict of the area tested by neutralization/inhibition.
1 - Pelvis level [13,15] - Figure 2A
With the subject in the supine position, an asymmetry of the position of the pubic rami was sought. If that was the case, after having determined the relative positions of the pubic rami by palpation, the thigh, on the side with the lower ramus, was placed at maximum but nonpainful flexion of the pelvis, with the leg on the thigh [32]. The clinician placed his chest on the bent knee and a hand on the contralateral knee, and then simultaneously opposed the extension of the thigh on the pelvis and the contralateral crural flexion, while the patient contracted muscles without pain, for at least 6 seconds, to counter the action [13,15]. This maneuver was repeated three times.
2 - Oropharynx level [13,15] – Figure 2B
This corresponds to disruption and incoordination of swallowing muscles. Specific proprioceptive physiotherapy at the oropharynx level was carried out: the subject standing erect placed the tip of his/her tongue between his/her incisors and pinched it to maintain this position and swallowed 3 times while the clinician verified by palpation the movements associated with the hyoid bone [13,15]. After which the Maddox Rod Test was repeated.
3 - Dental occlusion level [13,15] – Figure 2C
With the subject in an upright stance, occlusion of the dental cusps was prevented by filling, if necessary, the spaces left resulting from missing teeth, and/or by placing a 10x38 mm–dental cotton rolls between the teeth first bilaterally [34] and then unilaterally if the former was ineffective [15]. After biting 2 or 3 times, the subject stopped biting before performing the Maddox Rod Test (i.e. natural posture without added voluntary task). If the VO was obtained, when the dental cotton rolls were removed, VH reappeared. Then, usually the same result (i.e. VO) could be observed after specific proprioceptive physiotherapy at the TMJ level, i.e. mandibular movements as translation, protraction, depression, and elevation [13,15,35,36]. This can help the subject until consulting a dentist/stomatologist.
Note that in rare cases, subjects with VO were able to present VH consecutive to a slight dental intercuspidation (25 subjects, 95% CI 2.6 – 5.8), an interaction already known on postural muscle tone [37], requiring the advice of a specialist.
4 - Plantar afferences level [16] – Figure 2D
To test the plantar afferences, the thin and firm foam was used between the ground and the feet to focus the action on plantar cutaneous afferents; Garrigues [16] sometimes found a cancellation of VH present in dyslexics. This procedure decreased the information arising from the feet [38-44]. If the VO was obtained after the subject had trampled 4 to 5 times on the foam and stopped, when the foam was removed, VH reappeared. Then, the same result (i.e. VO) could be observed after specific proprioceptive physiotherapy at the foot level as eversion and inversion, and flexion and extension of the toes [45]. This can help the subject until consulting a podiatrist.
5 - Piercings with jewelry [8]
Removing body piercings (e.g. jewelry pierced in the eyebrow, tragus, upper lip, nostril [8], or umbilicus) except those of the lobes of the ears, could be enough to obtain VO. This suggested that the subject should not put them back. Note that if one cannot remove it/them, one can use cold spray on its/their place(s) with transient effect, but sufficiently for the test.
6 - Vision [46]– Figure 2E
At this step, when the bilateral VO was not achieved, this could be due to an interaction involving vision. Here, after the subject kept his/her eyes closed for 30 seconds [46,47] one had to test and check the amplitude of movement with eyes still closed and observe an improvement of the mobility of spinal and peripheral joints after initial alternation eyes open [46]- for example through active head rotation on the trunk and a relative symmetrical rotation of the head. The limitation of the amplitude on one side immediately reappears when the subject opens her/his eyes. Then, for the subjects who had glasses, mobility tests were repeated with their eyes open, and VH could be tested again with the Maddox Rod Test: most of the time the improvement was observed, and it was suggested to wear the spectacles all the time, but was not always the case; sometimes the improvement was achieved with the glasses “straightened” and suggested a check of the frame by the optometrist/optician. If the subject did not have visual correction (or a persistent visual problem suspected despite glasses), the hypothesis of a visual input disorder was advanced and suggests consulting a vision specialist. The same effect (i.e. eyes open), but transient, as eyes closed on mobility, was obtained by applying pressure to the reflexed tendon of the obliquus superior (eye) muscles or the supraorbital nerve [35], accompanied by immediate relief of pain, if any [46].
Figure 2: Pelvis (A), oropharynx (B), dental occlusion (C), plantar afferences (D), and vision (E) levels for their specific investigations and maneuvers.
From this clinical investigation using the Maddox Rod Test to detect mild vertical heterophoria and seek sensory conflicts, the results of the 525 subjects’ data analysis found the following: the pelvis level was found in 236 subjects (95% CI 40.6 – 49.2), the oropharynx level in 221 (95% CI 37.9 – 46.3), the dental occlusion level (95% CI 41.2 – 49.8), the plantar afferences level (95% CI 3.5 – 7.5), piercings with jewelry (95% CI 3.9 – 7.9), and vision level (95% CI 26.4 – 34.2). Rates and number of participants observed in the tested sample for the six levels are presented in Table 1. Ninety-five percent confidence intervals (CI) are derived from the data of 525 subjects mentioned above and have only an indicative value specific to the place and the population investigated.
This retrospective study reports that VH can be linked to conflict from the stomatognathic system, the pelvis, plantar afferences, piercings if any, or/and from visual problems (Table 1). These conflicts may arise from a disturbance in afferences from somesthesia cues and/or vision cues. At the pelvis and oropharynx level (steps 1 and 2), the same maneuvers used for therapeutic purposes were also used for the diagnosis. In the subsequent steps, neutralizing these afferences (steps 3 to 5, i.e. dental cotton rolls, foam under the feet, jewelry removed) could make the VH disappear or reappear immediately, along with the tonic asymmetries (as in step 6, with or without glasses, eyes open and eyes closed).
| Table 1: Number of participants, rate, and confidence interval observed in the tested sample for each location. | |||
| Location | Number | Rate (%) | Confidence Interval |
| Pelvis | 236 | 44,9 | [40,7 - 49,2] |
| Oropharynx | 221 | 42,1 | [37,9 - 46,3] |
| Dental Occlusion | 239 | 45,5 | [41,3 - 49,8] |
| Plantar afferences | 29 | 5,5 | [3,6 - 7,5] |
| Piercings with jewelry | 31 | 5,9 | [3,9 - 7,9] |
| Vision | 159 | 30,3 | [26,4 - 34,2] |
There are muscular synergies between extraocular muscles, neck, trunk, and lower limb muscles, more generally for postural responses [47,48]. As previously mentioned, vision and gaze signals are known to be involved with vestibular and somaesthetic inputs, in the sensorimotor/perception-action loops for posture, balance, movement control, and in the tonic activity in Humans [9,10,47] via the vestibulo-ocular, the vestibulospinal and the reticulospinal system [49]. Somaesthetic signals are required for eye movement and the visual perception of the surrounding space. For instance, Roll and Roll [48] showed that for a subject in an upright stance looking at a small target in darkness, the vibrations applied to the eye, the neck, or the leg elicited an illusory target displacement in the lateral direction, as in the case of masseter and temporalis muscles [50]. Applying vibratory stimulations to masseter and temporalis muscles elicited such visual displacement in the vertical plane in subjects with VH, but not in subjects with VO, suggesting that these muscles were part of the lateral muscle proprioceptive chain and that proprioceptive dysfunction could interact on the antero-posterior chain [50].
Modifying signal/afferent at a given location, voluntarily or not, by an internal or external stimulation, immediately the muscle response/the postural tone distribution physiologically changes on the whole body (e.g. direction of gaze, head position, a prism in front of one eye, stimulation of the plantar afferences) [19,47,48,51,52]. For instance, modifying sensory information such as visual cues or gaze direction induces a modification of the tone repartition from the eye to the lower limbs [53,54].
Postural dysfunctions are found in nonspecific back pain, headache, dizziness, tinnitus, postural deficiency syndrome, or tendinopathies [55-62]. Furthermore, in these pathologies and others such as temporomandibular joint disorder, mild traumatic brain injuries or in the presence of small VH, similar comorbidity can exist – as back pain, musculoskeletal and abdominal pain, headache, dizziness, tinnitus, eyestrain [8,11,63-66] This is in agreement with experimental pain models where induced sensorimotor conflict between vision and somaesthetic signals in healthy subjects could quickly lead to these kinds of symptoms, or the discordance between motor intention, and proprioceptive cues and visual signals could lead to pain [3,4].
Beyond the global muscle tone in the gravity field (more or less general stiffness, hypertonicity/hypotonicity), when a sensory conflict initially exists (e.g. from vision, stomatognathic system, plantar afferences), it can lead to lower motor control and induce asymmetric postural tone [67-69].
It is also from these labile and organized asymmetries that clinical postural investigation was born, particularly with Gagey, et al. [70] in the context of post-concussion syndrome. These asymmetries should be distinguished from the anatomical and behavioral asymmetries which cannot immediately change [67,71]. Gagey, et al. [71] demonstrated that when a subject looks straight ahead at a target, with arms at shoulder level in front of her/him during standing, they exhibit a slight rotation and contralateral translation of the body after closing her/his eyes. Additionally, electromyography recordings from the lower limbs revealed an asymmetrical activity between both sides with eyes open, which decreased with eyes closed [72]. This suggests that the subject balances on internal forces and basic tone, no longer being influenced by the direction of gaze straight ahead of being “in a spin”. This means that when the subject’s eyes open, whether to remain oriented facing a target, a scene of interest, for walking, running, or other dynamic activities, unconsciously, an added effort of counter-rotation is necessary to remain facing or maintain the trajectory and suggests rotational constraints. Particularly, this can be understood in the context of sports or work with musculoskeletal disorders. Indeed, the overuse of tendon-muscular complexes is known to be able to lead to pain, injury, to non-contact injuries (e.g. for reviews, see e.g. Shepherd & Screen, 2013; Martin, et al. 2018). To give an image, it is the subject in a car with a lack of parallelism, the eyes would be the steering wheel. When asymmetric postural tone exists, i.e. in the presence of more or less strong muscular activity, the permanent muscular tension on an injured site is known to slow down / hinder healing [73,74].
In nonspecific chronic pain subjects with comorbidities (such as dizziness, tinnitus), VH most of the time exists, with asymmetric postural tone [7,19,30,75,76]. Jackson and Bedell [77] found an association between vertical phoria and motion sickness/dizziness, but suggest the relationship may not be causal. VH is not prejudged as regards the origin, and canceling the conflict from somaesthetic disorder or/and from vision can restore VO immediately. Previously studies have shown in these cases that the restoration of VO could be accompanied by a reduction in pain in the following days and an improvement in motor control, the mobility of spinal and peripheral joints, and performance in the balance tests after initial alternation [12,13].
So, the present proposed management of non-contact injuries, nonspecific chronic pain, and prevention via sensory conflict detection with vertical heterophoria as a landmark indicator can be understood in this context.
Supplementary
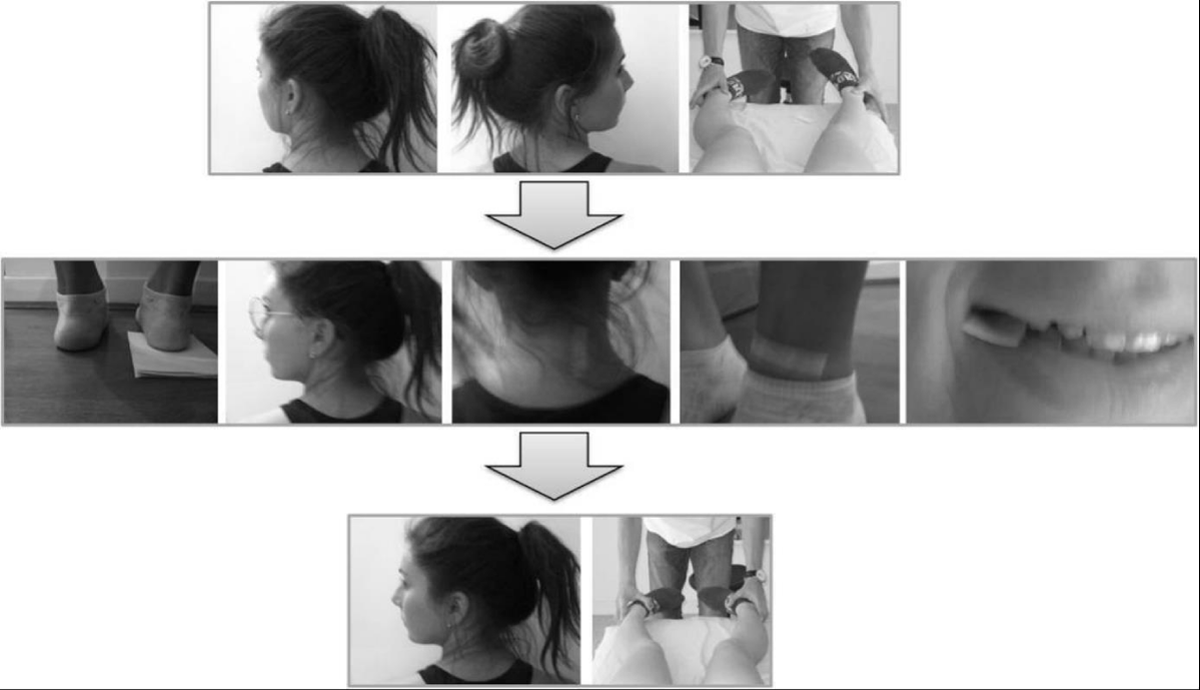
Note the impact on the tonic asymmetries, for instance at the cervical spine level where head rotation should be limited on one side – e.g. left side – when VH is present (in patients with nonspecific chronic pain or in healthy subjects, [12,13,15,19]), this limitation can be canceled for the same subject with different stimulated areas (the reader will be able to easily verify at home/on patients): e.g. a piece of adhesive tape (skin stimulation) on the right side of the neck, or behind the left knee, or in front of the left ankle, as a sheet of paper folded in four under the right heel (foot stimulation), a little piece of paper between two teeth (upper and lower – stomatognathic stimulation) on the right or 1-diopter prism base in placed on the left eye (visual stimulation) –(Figure 3). Removing the stimulation, the limitation immediately reappeared. This gain with different stimulated areas should not be confused with the gain on postural control /range of motion achieved with inhibition/neutralization from different cues; that could perhaps lead to an error of interpretation and nonoptimal management – as for subjects with VH and piercings (jewelry) suffering from nonspecific symptoms: VH can be canceled by an appropriate vertical prism (visual stimulation) versus removing the jewelry [7]. Indeed, if the subject only has an interaction of dental occlusion, with for instance a limitation of the head rotation to the right side, the dental cotton rolls canceling VH (neutralization/inhibition) should cancel the limitation of the head rotation, through neither the foam under the feet nor eyes closed would.
Figure 3: Active head rotation with limitation on the left side, and passive hip internal rotation on the right side. The same gain with one of the different stimulated areas – for example under the heel, a one-diopter prism in front of one eye, an adhesive tape on the skin, or a little piece of paper between teeth. See text for details.
Empirically, in clinical practice, we observe the possible causes inducing: the lack of pelvic static which could be due to a fall, childbirth, or bad spine health behavior; oropharynx dysfunction which could be a bump to the head, traumatic brain injury, whiplash injury, otorhinolaryngology problems (past or present, e.g. sinusitis, thyroid) or history of surgical intervention with intubation; dental occlusion disorders likely on elements added as dental amalgam, crown or implant, dental prosthesis, or related to the missing tooth (or teeth, except all wisdom teeth) requiring consultation with a dentist or stomatologist; visual problem, generally for at least slight astigmatism and/or mild hypermetropia, already known in the context of mild VH [11,14].
Note that it cannot be said that all the disturbances of the described areas necessarily induce VH. For instance, one can have a dental malocclusion or a piercing without VH.
Prevalence and henceforth the confidence intervals are those related to this clinical investigation in various sites, for different purposes for which we are asked (i.e. to find leads and treat chronic pain and other nonspecific symptoms, for prevention, and/or for performance); does not mean that it is the generality. These investigations, within the decision tree, using the Maddox Rod Test when practicable for VH detection remain a potential landmark in the management of nonspecific chronic pain, non-contact injuries, and prevention, and so give us trackers. Further clinical research would be of interest on different pathologies.
For the pelvis level, a test for symphysiolysis detection is validated, involving pain reproduction during palpation [78,79], but the literature did not support the palpation of pubic rami to detect a positional asymmetry. To avoid this limitation, the clinician could consider a priori the pelvic implication in the sensorial conflict and apply a “bilateral saturation” technique, i.e. as described for “1-Pelvis level” on each side.
When the Maddox Rod Test detected a mild VH in upright reference posture in humans, an afference disorder was likely, but not prejudged as regards the origin. It could be used as a landmark in which sensory afferent conflict could induce symptoms and/or a non-optimal postural control / a lower motor efficiency. This provided informative indications of the therapeutic intervention as a decision tree based on the disappearance of the VH by inhibition in an etiological way, indicating the area(s) to be treated, its persistence points towards a persistent vision defect.
The proposed protocol is inscribed in the field of clinical posturology investigation [70,80] and suggests a pluridisciplinary approach and could contribute to potentiate/complete other investigations, such as those already based on vertical phoria or not. Further clinical research is needed.
The present article is written in tribute to Dr. Bernard Weber and Dr. Pierre-Marie Gagey who asked to share this. The author thanks Alexandre Kubicki for his help, Clodagh Geoghegan for English revision, and thanks the anonymous reviewers for their constructive comments.
Conflict of interest disclosure
The authors have no financial or proprietary interest in materials or procedure presented herein. All the recruited patients gave their informed consent about the use of their evaluation data.
- Waddell G. 1987 Volvo award in clinical sciences. A new clinical model for the treatment of low-back pain. Spine (Phila Pa 1976). 1987 Sep;12(7):632-44. doi: 10.1097/00007632-198709000-00002. PMID: 2961080.
- Hancock MJ, Maher CG, Laslett M, Hay E, Koes B. Discussion paper: what happened to the ‘bio’ in the bio-psycho-social model of low back pain? Eur Spine J. 2011 Dec;20(12):2105-10. doi: 10.1007/s00586-011-1886-3. Epub 2011 Jun 25. PMID: 21706216; PMCID: PMC3229745.
- Harris AJ. Cortical origin of pathological pain. Lancet. 1999 Oct 23;354(9188):1464-6. doi: 10.1016/S0140-6736(99)05003-5. PMID: 10543687.
- McCabe CS, Haigh RC, Halligan PW, Blake DR. Simulating sensory-motor incongruence in healthy volunteers: implications for a cortical model of pain. Rheumatology (Oxford). 2005 Apr;44(4):509-16. doi: 10.1093/rheumatology/keh529. Epub 2005 Jan 11. PMID: 15644392.
- McCabe CS, Cohen H, Blake DR. Somaesthetic disturbances in fibromyalgia are exaggerated by sensory motor conflict: implications for chronicity of the disease? Rheumatology (Oxford). 2007 Oct;46(10):1587-92. doi: 10.1093/rheumatology/kem204. Epub 2007 Sep 1. PMID: 17767000.
- Matheron E, Kapoula Z. Vertical heterophoria and postural control in nonspecific chronic low back pain. PLoS One. 2011 Mar 30;6(3):e18110. doi: 10.1371/journal.pone.0018110. PMID: 21479210; PMCID: PMC 3068140.
- Matheron E, Kapoula Z. Face Piercing (Body Art): Choosing Pleasure vs. Possible Pain and Posture Instability. Front Physiol. 2011 Sep 21;2:64. doi: 10.3389/fphys.2011.00064. PMID: 21960975; PMCID: PMC3177080.
- Matheron E, Kapoula Z. Incidence of vertical phoria on postural control during binocular vision: what perspective for prevention to nonspecific chronic pain management? Med Hypothesis Discov Innov Ophthalmol. 2015 Spring;4(1):27-30. PMID: 25861672; PMCID: PMC4389295.
- Nashner LM. Adapting reflexes controlling the human posture. Exp Brain Res. 1986; 26: 59-72.
- Roll JP, Vedel JP, Roll R. Eye, head and skeletal muscle spindle feedback in the elaboration of body references. Prog Brain Res. 1989;80:113-23; discussion 57-60. doi: 10.1016/s0079-6123(08)62204-9. PMID: 2634269.
- Amos FJ, Rutstein R. Vertical deviation. In F.J. Amos (Ed), Diagnosis and management in vision care. 1987; 515-583. Amsterdam, New-York, Oxford: Butterworths.
- Matheron E, Quercia P, Weber B, Gagey PM. Vertical heterophoria and postural deficiency syndrome. Gait Posture. 2005a; 21(suppl 1):132-133.
- Matheron E, Barlaud P, D’Athis P. [Evaluation of vertical heterophoria in distance vision in so-called chronic arthralgic and/or spinal pain subjects, and incidence of their normalization by specific proprioceptive physiotherapy]. In M. Lacour, & B. Weber (Eds). [Bipedalism, postural control and cortical representation]. Marseille, Solal. 2005b; 213-220.
- Scheiman M, Wick B. Clinical management of binocular vision, heterophoric, accommodative and eye movement disorders. Philadelphia, Lippincott. 1994.
- Matheron E. [Vertical heterophoria and myotonic normalisation]. Kinésithér Scient. 2000; 34: 23-28.
- Garrigues B. [Visual spatial localization abnormalities in dyslexic children. Preliminary study]. In B. Weber, & P. Villeneuve (Eds). [Clinical posturology: motor and cognitive dysfunctions]. Paris, Elsevier Masson. 2007; 70-75.
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019 Jan;160(1):19-27. doi: 10.1097/j.pain.0000000000001384. PMID: 30586067.
- Matheron E, Kapoula Z. Vertical phoria and postural control in upright stance in healthy young subjects. Clin Neurophysiol. 2008 Oct;119(10):2314-20. doi: 10.1016/j.clinph.2008.06.016. Epub 2008 Aug 28. PMID: 18760665.
- Matheron E, Zandi A, Wang D, Kapoula Z. A 1-Diopter Vertical Prism Induces a Decrease of Head Rotation: A Pilot Investigation. Front Neurol. 2016 Apr 28;7:62. doi: 10.3389/fneur.2016.00062. PMID: 27199886; PMCID: PMC4848294.
- Daum KM. Heterophoria and heterotropia. In J.B. Eskridge, F.J. Amos, & J.D. Barlett (Eds). Clinical procedures in optometry. Philadelphia, J.B. Lippincott Compagny. 1991; 72-90.
- Wong AM, Tweed D, Sharpe JA. Vertical misalignment in unilateral sixth nerve palsy. Ophthalmology. 2002 Jul;109(7):1315-25. doi: 10.1016/s0161-6420(02)01067-9. PMID: 12093657.
- Casillas Casillas E, Rosenfield M. Comparison of subjective heterophoria testing with a phoropter and trial frame. Optom Vis Sci. 2006 Apr;83(4):237-41. doi: 10.1097/01.opx.0000214316.50270.24. PMID: 16614580.
- Matheron E, Yang Q, Lê TT, Kapoula Z. Effects of ocular dominance on the vertical vergence induced by a 2-diopter vertical prism during standing. Neurosci Lett. 2008 Oct 24;444(2):176-80. doi: 10.1016/j.neulet.2008.08.025. Epub 2008 Aug 14. PMID: 18718507.
- Schor CM, McCandless JW. An adaptable association between vertical and horizontal vergence. Vision Res. 1995 Dec;35(23-24):3519-27. doi: 10.1016/0042-6989(95)00063-k. PMID: 8560816.
- Von Noorden GK, Campos EC. Binocular vision and ocular motility: theory and management of strabismus. 6th ed. St.Louis, Mosby. 2002.
- Matheron E. [Incidence of vertical phoria on postural control during binocular vision]. Doctoral dissertation, University of Paris V – René Descartes. Paris, France. 2009.
- Ungerleider LG, Mishkin M. Two cortical visual systems. In D.J. Ingle, M.A. Goodale, & Mansfield, R.J.W. (Eds) Analysis of visual behaviour. Cambridge, MA, MIT Press. 1982; 549-586
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992 Jan;15(1):20-5. doi: 10.1016/0166-2236(92)90344-8. PMID: 1374953.
- Amblard B, Crémieux J, Marchand AR, Carblanc A. Lateral orientation and stabilization of human stance: static versus dynamic visual cues. Exp Brain Res. 1985;61(1):21-37. doi: 10.1007/BF00235617. PMID: 4085597.
- Quercia P, Quercia M, Feiss LJ, Allaert F. The distinctive vertical heterophoria of dyslexics. Clin Ophthalmol. 2015 Sep 25;9:1785-97. doi: 10.2147/OPTH.S88497. PMID: 26445526; PMCID: PMC4590632.
- Rosner MS, Feinberg DL, Doble JE, Rosner AJ. Treatment of vertical heterophoria ameliorates persistent post-concussive symptoms: A retrospective analysis utilizing a multi-faceted assessment battery. Brain Inj. 2016;30(3):311-7. doi: 10.3109/02699052.2015.1113564. Epub 2016 Feb 1. PMID: 26829465.
- Niboyet JEH. [Practice of manual medicine]. Paris, Maisonneuve publisher. 1968.
- Clauzade MA, Darraillans B. [Osteopathic concept of occlusion]. Perpignan, SEOO publisher. 1989.
- Meersseman JP, Esposito GM. [Evaluation of the relationship between occlusion and posture]. Modern Dentist. 1988; 6: 5-69.
- Bourdiol RJ. [Reflex cephalic neurotherapy]. Moulins-lès-Metz, Maisonneuve ed. 1987.
- Bourdiol RJ, Bortolin G. [Headache HemicraniE – New nosological classification and multidisciplinary therapeutic approach]. Treviso, Gemmer Italia, Edizioni, Guia, Valdobbiadene. 2000.
- Bonnier L. Eighth lesson. In PM Gagey, G Bizzo, L Bonnier, R Gentaz, P Guillaume, C Marucchi, P Villeneuve (Eds), [Eight lessons]. 4th ed. Paris, French Association of Posturology. 1993.
- Bles W, De Wit G. Study of the effects of optic stimuli on standing. Agressologie. 1976;17(C Spec No):1-5. PMID: 1008135.
- Dujols A. [The plantar quotiens and visual-podal conflict]. Agressologie. 1996; 32: 192–194.
- Lepork AM, Villeneuve P. [Irritating plantar support thorns: clinical and stabilometric observations]. In P. Villeneuve (Ed). [Foot, Balance and Posture]. Paris, Frison-Roche. 1986; 131-38.
- Yi Y, Park S. Effect of reduced cutaneous cues on motion perception and postural control. Exp Brain Res. 2009 May;195(3):361-9. doi: 10.1007/s00221-009-1796-3. Epub 2009 Apr 29. PMID: 19404630.
- Janin M. [The plantar sensitivity and plantar motility: their influence on posturo-kinetics control activities in healthy and pathological subjects]. Doctoral dissertation, Unive University of Toulouse III – Paul Sabatier. Toulouse, France. 2009.
- Foisy A. [The role of plantar cutaneous afferents in postural and oculomotor control in healthy subjects and in subjects with a nonsymptomatic plantar exteroceptive inefficiency]. Doctoral dissertation, University of Paris V – René Descartes, Paris, France. 2016.
- Foisy A, Kapoula Z. How Plantar Exteroceptive Efficiency Modulates Postural and Oculomotor Control: Inter-Individual Variability. Front Hum Neurosci. 2016 May 13;10:228. doi: 10.3389/fnhum.2016.00228. PMID: 27242490; PMCID: PMC4866577.
- Bourdiol RJ. [Podo-reflexo-kinesiology]. Moulins-lès-Metz, Maisonneuve ed. 1986.
- Matheron E, Weber B. [Involvement of visual input in tonic postural asymmetries: clinical approach]. In D. Perennou, & M. Lacour (Eds). [Efficiency and deficiencies of postural control]. Marseille, Solal. 2006; 261-270.
- Ivanenko Y, Grasso RF, Lacquaniti. Effect of gaze on postural responses to neck proprioceptive and vestibular stimulation in humans. Journal of Physiology. 1999; 519(1): 301-314.
- Roll JP, Roll R. From eye to foot: a proprioceptive chain involded in postural control. In B. Amblard, A. Berthoz, & F. Clarac (Eds). Posture and Gait: Development, Adaptation, and Modulation. Amsterdam, Elseiver. 1988; 155-164.
- Berthoz A. The role of gaze in compensation of vestibular disfunction: the gaze substitution hypothesis. Prog Brain Res. 1988;76:411-20. doi: 10.1016/s0079-6123(08)64528-8. PMID: 3064159.
- Matheron E, Mourey F, Weber B. [Vertical heterophoria can be modified by vibration of the masseter and temporalis tendons]. In M. Lacour (Ed). [New posturographic signal processing methods]. Solal, Marseille. 2004; 145-152
- Janin M, Dupui P. The effects of unilateral medial arch support stimulation on plantar pressure and center of pressure adjustment in young gymnasts. Neurosci Lett. 2009 Sep 25;461(3):245-8. doi: 10.1016/j.neulet.2009.06.043. Epub 2009 Jun 21. PMID: 19545613.
- Bonaventura RE, Giustino V, Chiaramonte G, Giustiniani A, Smirni D, Battaglia G, Messina G, Oliveri M. Investigating prismatic adaptation effects in handgrip strength and in plantar pressure in healthy subjects. Gait Posture. 2020 Feb;76:264-269. doi: 10.1016/j.gaitpost.2019.12.022. Epub 2019 Dec 23. PMID: 31881480.
- Gagey PM, Baron JB, Lespargot J, Poli JP. [Variations of postural tonic activity and the activity of oculocephalogyric muscles in cathedrostatism]. Agressologie. 1973;14 Spec B(0):87-95. French. PMID: 4802358.
- Gagey PM. [The law of the canals]. Agressologie. 1988 Oct;29(10):691-2. French. PMID: 3247894.
- Da Cunha HM, Da Silva OA. [Postural deficiency syndrome. Its importance in ophthalmology]. J Fr Ophtalmol. 1986;9(11):747-55. French. PMID: 3571839.
- Leetun DT, Ireland ML, Willson JD, Ballantyne BT, Davis IM. Core stability measures as risk factors for lower extremity injury in athletes. Med Sci Sports Exerc. 2004 Jun;36(6):926-34. doi: 10.1249/01.mss.0000128145.75199.c3. PMID: 15179160.
- Mok NW, Brauer SG, Hodges PW. Hip strategy for balance control in quiet standing is reduced in people with low back pain. Spine (Phila Pa 1976). 2004 Mar 15;29(6):E107-12. doi: 10.1097/01.brs.0000115134.97854.c9. PMID: 15014284.
- Dros J, Maarsingh OR, Beem L, van der Horst HE, ter Riet G, Schellevis FG, van Weert HC. Impact of dizziness on everyday life in older primary care patients: a cross-sectional study. Health Qual Life Outcomes. 2011 Jun 16;9:44. doi: 10.1186/1477-7525-9-44. PMID: 21679451; PMCID: PMC3142198.
- Kapoula Z, Yang Q, Lê TT, Vernet M, Berbey N, Orssaud C, Londero A, Bonfils P. Medio-lateral postural instability in subjects with tinnitus. Front Neurol. 2011 May 27;2:35. doi: 10.3389/fneur.2011.00035. PMID: 21647364; PMCID: PMC3103995.
- De Blaiser C, Roosen P, Willems T, Danneels L, Bossche LV, De Ridder R. Is core stability a risk factor for lower extremity injuries in an athletic population? A systematic review. Phys Ther Sport. 2018 Mar;30:48-56. doi: 10.1016/j.ptsp.2017.08.076. Epub 2017 Aug 24. PMID: 29246794.
- Scholes M, Stadler S, Connell D, Barton C, Clarke RA, Bryant AL, Malliaras P. Men with unilateral Achilles tendinopathy have impaired balance on the symptomatic side. J Sci Med Sport. 2018 May;21(5):479-482. doi: 10.1016/j.jsams.2017.09.594. Epub 2017 Oct 6. PMID: 29054749.
- Carvalho GF, Schwarz A, Szikszay TM, Adamczyk WM, Bevilaqua-Grossi D, Luedtke K. Physical therapy and migraine: musculoskeletal and balance dysfunctions and their relevance for clinical practice. Braz J Phys Ther. 2020 Jul-Aug;24(4):306-317. doi: 10.1016/j.bjpt.2019.11.001. Epub 2019 Nov 29. PMID: 31813696; PMCID: PMC7351966.
- Baron JB, Goumot H, Gagey PM, Filliozat R, Gentaz R, Koitcheva V, Rouquet Y, Fouque A, Bessineton JC, Pacifici M, Ushio N. [Disturbance of tonic postural activity of oculomotor origins due to head injury. Neuro-ophthalmological and pharmacological aspects]. Agressologie. 1975;16 Spec No D:53-64. French. PMID: 1085575.
- Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005 Feb;113(3):331-339. doi: 10.1016/j.pain.2004.11.010. PMID: 15661441.
- Doble JE, Feinberg DL, Rosner MS, Rosner AJ. Identification of binocular vision dysfunction (vertical heterophoria) in traumatic brain injury patients and effects of individualized prismatic spectacle lenses in the treatment of postconcussive symptoms: a retrospective analysis. PM R. 2010 Apr;2(4):244-53. doi: 10.1016/j.pmrj.2010.01.011. PMID: 20430325.
- Wiesinger B, Malker H, Englund E, Wänman A. Back pain in relation to musculoskeletal disorders in the jaw-face: a matched case-control study. Pain. 2007 Oct;131(3):311-319. doi: 10.1016/j.pain.2007.03.018. Epub 2007 Apr 24. PMID: 17459585.
- Guillaume P. [The clinical postural examination]. Agressologie. 1988; 29(10): 687-690.
- Gagey PM, Weber B. [Posturology: Regulation and standing disturbances]. 3ed, Masson. 2004.
- Alexandre M, Anne-Marie B, Valérie J, Yves B, Patrick Q. Are changes in the stomatognatic system able to modify the eye balance in dyslexia? J Oral Biol Craniofac Res. 2019 Apr-Jun;9(2):166-171. doi: 10.1016/j.jobcr.2019.03.005. Epub 2019 Mar 24. PMID: 30976507; PMCID: PMC 6439284.
- Gagey PM, Baron JB, Ushio N. [Introduction to clinical posturology]. Agressologie. 1980;21(E):119-23. French. PMID: 7246889.
- Gagey PM, Asselain B, Ushio N, Baron JB. [Asymmetries of orthostatic posture: are they due to chance?]. Agressologie. 1977;18(5):277-83. French. PMID: 602990.
- Gentaz R, Asselain B, Lévy J, Gagey PM. [Electromyographic approach to orthostatic posture asymmetries]. Agressologie. 1979; 20(B): 113-114.
- Paxton JZ, Grover LM, Baar K. Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng Part A. 2010 Nov;16(11):3515-25. doi: 10.1089/ten.TEA.2010.0039. Epub 2010 Aug 28. PMID: 20593972.
- Paxton JZ, Hagerty P, Andrick JJ, Baar K. Optimizing an intermittent stretch paradigm using ERK1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A. 2012 Feb;18(3-4):277-84. doi: 10.1089/ten.TEA.2011.0336. Epub 2011 Dec 22. PMID: 21902469; PMCID: PMC3267962.
- Quercia P, Seigneuric A, Chariot S, Vernet P, Pozzo T, Bron A, Creuzot-Garcher C, Robichon F. [Ocular proprioception and developmental dyslexia. Sixty clinical observations]. J Fr Ophtalmol. 2005 Sep;28(7):713-23. French. doi: 10.1016/s0181-5512(05)80983-0. PMID: 16208221.
- Matheron E. [Maddox Rod Test (vertical streaks) and postural deficiency syndrome]. In B Weber, P Villeneuve (Eds) [Clinical posturology: motor and cognitive dysfunctions]. Elsevier Masson, Paris. 2007; 44-51.
- Jackson DN, Bedell HE. [Vertical heterophoria and susceptibility to visually induced motion sickness]. Clinical posturology: motor and cognitive dysfunctions] Strabismus. 2012 Mar;20(1):17-23. doi: 10.3109/09273972.2011.650813. PMID: 22390327; PMCID: PMC 3625960.
- Albert H, Godskesen M, Westergaard J. Evaluation of clinical tests used in classification procedures in pregnancy-related pelvic joint pain. Eur Spine J. 2000 Apr;9(2):161-6. doi: 10.1007/s005860050228. PMID: 10823434; PMCID: PMC3611366.
- Hansen A, Jensen DV, Larsen EC, Wilken-Jensen C, Kaae BE, Frølich S, Thomsen HS, Hansen TM. Postpartum pelvic pain--the “pelvic joint syndrome”: a follow-up study with special reference to diagnostic methods. Acta Obstet Gynecol Scand. 2005 Feb;84(2):170-6. doi: 10.1111/j.0001-6349.2005.00687.x. PMID: 15683379.
- Gagey PM. A critique of posturology: towards an alternative neuro-anatomy? Surg Radiol Anat. 1991;13(4):255-7. doi: 10.1007/BF01627752. PMID: 1803533.